Experimental methodologies and evaluations of computer-aided drug design methodologies applied to a series of 2-aminothiophene derivatives with antifungal activities
- PMID: 22367025
- PMCID: PMC6269054
- DOI: 10.3390/molecules17032298
Experimental methodologies and evaluations of computer-aided drug design methodologies applied to a series of 2-aminothiophene derivatives with antifungal activities
Abstract
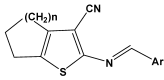
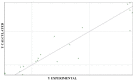
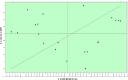
Fifty 2-[(arylidene)amino]-4,5-cycloalkyl[b]thiophene-3-carbonitrile derivatives were screened for their in vitro antifungal activities against Candida krusei and Cryptococcus neoformans. Based on experimentally determined minimum inhibitory concentration (MIC) values, we conducted computer-aided drug design studies [molecular modelling, chemometric tools (CPCA, PCA, PLS) and QSAR-3D] that enable the prediction of three-dimensional structural characteristics that influence the antifungal activities of these derivatives. These predictions provide direction with regard to the syntheses of new derivatives with improved biological activities, which can be used as therapeutic alternatives for the treatment of fungal infections.
Figures

Similar articles
-
Antifungal activity of topical microemulsion containing a thiophene derivative.Braz J Microbiol. 2014 Aug 29;45(2):545-50. doi: 10.1590/s1517-83822014000200024. eCollection 2014. Braz J Microbiol. 2014. PMID: 25242940 Free PMC article.
-
Preliminary antifungal and cytotoxic evaluation of synthetic cycloalkyl[b]thiophene derivatives with PLS-DA analysis.Acta Pharm. 2012 Jun;62(2):221-36. doi: 10.2478/v10007-012-0017-y. Acta Pharm. 2012. PMID: 22750820
-
Comparative study of antifungal activity of sertaconazole, terbinafine, and bifonazole against clinical isolates of Candida spp., Cryptococcus neoformans and dermatophytes.Chemotherapy. 1997 Nov-Dec;43(6):387-92. doi: 10.1159/000239596. Chemotherapy. 1997. PMID: 9395851
-
Screening of Natural Lead Molecules Against Putative Molecular Targets of Drug-resistant Cryptococcus spp: An Insight from Computer-aided Molecular Design.Curr Top Med Chem. 2018;18(31):2681-2701. doi: 10.2174/1568026619666190119145434. Curr Top Med Chem. 2018. PMID: 30659541 Review.
-
Recent contribution of medicinally active 2-aminothiophenes: A privileged scaffold for drug discovery.Eur J Med Chem. 2022 Aug 5;238:114502. doi: 10.1016/j.ejmech.2022.114502. Epub 2022 Jun 2. Eur J Med Chem. 2022. PMID: 35696863 Review.
Cited by
-
Editorial: In Silico Studies in Drug Research Against Neurodegenerative Diseases.Curr Neuropharmacol. 2018;16(6):647-648. doi: 10.2174/1570159X1606180608103840. Curr Neuropharmacol. 2018. PMID: 29938615 Free PMC article. No abstract available.
-
Chitosan-Based Films with 2-Aminothiophene Derivative: Formulation, Characterization and Potential Antifungal Activity.Mar Drugs. 2022 Jan 26;20(2):103. doi: 10.3390/md20020103. Mar Drugs. 2022. PMID: 35200633 Free PMC article.
-
Incorporation of 2-amino-thiophene derivative in nanoparticles: enhancement of antifungal activity.Braz J Microbiol. 2020 Jun;51(2):647-655. doi: 10.1007/s42770-020-00248-7. Epub 2020 Mar 5. Braz J Microbiol. 2020. PMID: 32141030 Free PMC article.
-
Antifungal activity of topical microemulsion containing a thiophene derivative.Braz J Microbiol. 2014 Aug 29;45(2):545-50. doi: 10.1590/s1517-83822014000200024. eCollection 2014. Braz J Microbiol. 2014. PMID: 25242940 Free PMC article.
-
Enhanced Efficacy of Thiosemicarbazone Derivative-Encapsulated Fibrin Liposomes against Candidiasis in Murine Model.Pharmaceutics. 2021 Mar 4;13(3):333. doi: 10.3390/pharmaceutics13030333. Pharmaceutics. 2021. PMID: 33806702 Free PMC article.
References
-
- Brown J.M. Fungal infections in bone marrow transplant patients. Curr. Opin. Infect. Dis. 2004;17:347–352. doi: 10.1097/01.qco.0000136935.13662.af. - DOI - PubMed
-
- Bergold A.M., Georgiadis S. New antifungic drugs: A review. Visão Acadêmica . 2004;5:159–172.
-
- Ram V.J., Goel A., Shukla P.K., Kapil A. Synthesis of thiophenes and thieno[3,2-c]pyran-4-ones as antileishmanial and antifungal agents. Bioorg. Med. Chem. Lett. 1997;7:3101–3106. doi: 10.1016/S0960-894X(97)10153-6. - DOI
-
- Chobot V., Buchta V., Jahodárová H., Pour M., Opletal L., Jahodár L., Harant P. Antifungal activity of a thiophenepolyine from Leuzeacarthamoides. Phytotherapy. 2003;74:288–290. - PubMed
-
- Wardakhan W.W., Louca N.A., Kamel M.M. The reaction of 2 Aminocyclohexeno[b]thiophene derivatives with ethoxycarbonylisothiocyanate: Synthesis of fused thiophene derivatives with antibacterial and antifungal activities. Acta Chim. Slov. 2007;54:229–241.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

