Homologous recombination via synthesis-dependent strand annealing in yeast requires the Irc20 and Srs2 DNA helicases
- PMID: 22367032
- PMCID: PMC3338270
- DOI: 10.1534/genetics.112.139105
Homologous recombination via synthesis-dependent strand annealing in yeast requires the Irc20 and Srs2 DNA helicases
Abstract
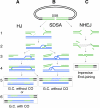
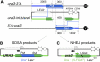
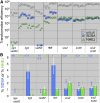
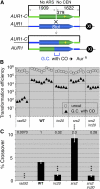
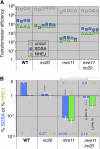
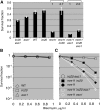
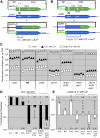
Synthesis-dependent strand-annealing (SDSA)-mediated homologous recombination replaces the sequence around a DNA double-strand break (DSB) with a copy of a homologous DNA template, while maintaining the original configuration of the flanking regions. In somatic cells at the 4n stage, Holliday-junction-mediated homologous recombination and nonhomologous end joining (NHEJ) cause crossovers (CO) between homologous chromosomes and deletions, respectively, resulting in loss of heterozygosity (LOH) upon cell division. However, the SDSA pathway prevents DSB-induced LOH. We developed a novel yeast DSB-repair assay with two discontinuous templates, set on different chromosomes, to determine the genetic requirements for somatic SDSA and precise end joining. At first we used our in vivo assay to verify that the Srs2 helicase promotes SDSA and prevents imprecise end joining. Genetic analyses indicated that a new DNA/RNA helicase gene, IRC20, is in the SDSA pathway involving SRS2. An irc20 knockout inhibited both SDSA and CO and suppressed the srs2 knockout-induced crossover enhancement, the mre11 knockout-induced inhibition of SDSA, CO, and NHEJ, and the mre11-induced hypersensitivities to DNA scissions. We propose that Irc20 and Mre11 functionally interact in the early steps of DSB repair and that Srs2 acts on the D-loops to lead to SDSA and to prevent crossoverv.
Figures

Similar articles
-
Putative antirecombinase Srs2 DNA helicase promotes noncrossover homologous recombination avoiding loss of heterozygosity.Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):16067-72. doi: 10.1073/pnas.1303111110. Epub 2013 Sep 16. Proc Natl Acad Sci U S A. 2013. PMID: 24043837 Free PMC article.
-
Heteroduplex DNA position defines the roles of the Sgs1, Srs2, and Mph1 helicases in promoting distinct recombination outcomes.PLoS Genet. 2013;9(3):e1003340. doi: 10.1371/journal.pgen.1003340. Epub 2013 Mar 14. PLoS Genet. 2013. PMID: 23516370 Free PMC article.
-
The Srs2 helicase activity is stimulated by Rad51 filaments on dsDNA: implications for crossover incidence during mitotic recombination.Mol Cell. 2008 Feb 1;29(2):243-54. doi: 10.1016/j.molcel.2007.11.033. Mol Cell. 2008. PMID: 18243118
-
Pathways and assays for DNA double-strand break repair by homologous recombination.Acta Biochim Biophys Sin (Shanghai). 2019 Sep 6;51(9):879-889. doi: 10.1093/abbs/gmz076. Acta Biochim Biophys Sin (Shanghai). 2019. PMID: 31294447 Review.
-
Multifunctional roles of Saccharomyces cerevisiae Srs2 protein in replication, recombination and repair.FEMS Yeast Res. 2017 Mar 1;17(2):fow111. doi: 10.1093/femsyr/fow111. FEMS Yeast Res. 2017. PMID: 28011904 Free PMC article. Review.
Cited by
-
Archaeal DNA Repair Mechanisms.Biomolecules. 2020 Oct 23;10(11):1472. doi: 10.3390/biom10111472. Biomolecules. 2020. PMID: 33113933 Free PMC article. Review.
-
Accurate and sensitive interactome profiling using a quantitative protein-fragment complementation assay.Cell Rep Methods. 2024 Oct 21;4(10):100880. doi: 10.1016/j.crmeth.2024.100880. Cell Rep Methods. 2024. PMID: 39437715 Free PMC article.
-
Regulation of hetDNA Length during Mitotic Double-Strand Break Repair in Yeast.Mol Cell. 2017 Aug 17;67(4):539-549.e4. doi: 10.1016/j.molcel.2017.07.009. Epub 2017 Aug 3. Mol Cell. 2017. PMID: 28781235 Free PMC article.
-
Putative antirecombinase Srs2 DNA helicase promotes noncrossover homologous recombination avoiding loss of heterozygosity.Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):16067-72. doi: 10.1073/pnas.1303111110. Epub 2013 Sep 16. Proc Natl Acad Sci U S A. 2013. PMID: 24043837 Free PMC article.
-
Heteroduplex DNA position defines the roles of the Sgs1, Srs2, and Mph1 helicases in promoting distinct recombination outcomes.PLoS Genet. 2013;9(3):e1003340. doi: 10.1371/journal.pgen.1003340. Epub 2013 Mar 14. PLoS Genet. 2013. PMID: 23516370 Free PMC article.
References
-
- Adams M. D., McVey M., Sekelsky J. J., 2003. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science 299: 265–267. - PubMed
-
- Bignon Y.-J., 2004. Biological basis of cancer predisposition, pp. 11–20 in Genetic Predisposition to Cancer, edited by Eeles R. A., Easton D. F., Ponder B. A. J., Eng C. Arnold, New York.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

