Characterization of bioaerosols from dairy barns: reconstructing the puzzle of occupational respiratory diseases by using molecular approaches
- PMID: 22367078
- PMCID: PMC3346481
- DOI: 10.1128/AEM.07661-11
Characterization of bioaerosols from dairy barns: reconstructing the puzzle of occupational respiratory diseases by using molecular approaches
Abstract
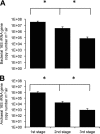
To understand the etiology of exposure-related diseases and to establish standards for reducing the risks associated with working in contaminated environments, the exact nature of the bioaerosol components must be defined. Molecular biology tools were used to evaluate airborne bacterial and, for the first time, archaeal content of dairy barns. Three air samplers were tested in each of the 13 barns sampled. Up to 10(6) archaeal and 10(8) bacterial 16S rRNA genes per m(3) of air were detected. Archaeal methanogens, mainly Methanobrevibacter species, were represented. Saccharopolyspora rectivirgula, the causative agent of farmer's lung, was quantified to up to 10(7) 16S rRNA genes per m(3) of air. In addition, a wide variety of bacterial agents were present in our air samples within the high airborne bioaerosol concentration range. Despite recommendations regarding hay preservation and baling conditions, farmers still develop an S. rectivirgula-specific humoral immune response, suggesting intense and continuous exposure. Our results demonstrate the complexity of bioaerosol components in dairy barns which could play a role in occupational respiratory diseases.
Figures

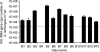

Similar articles
-
Culture-independent characterization of archaeal biodiversity in swine confinement building bioaerosols.Appl Environ Microbiol. 2009 Sep;75(17):5445-50. doi: 10.1128/AEM.00726-09. Epub 2009 Jun 26. Appl Environ Microbiol. 2009. PMID: 19561186 Free PMC article.
-
Detection of Saccharopolyspora rectivirgula by quantitative real-time PCR.Ann Occup Hyg. 2011 Jul;55(6):612-9. doi: 10.1093/annhyg/mer018. Epub 2011 Apr 21. Ann Occup Hyg. 2011. PMID: 21511892
-
Airborne microflora in Quebec dairy farms: lack of effect of bacterial hay preservatives.Am Ind Hyg Assoc J. 1999 Jan-Feb;60(1):89-95. doi: 10.1080/00028899908984426. Am Ind Hyg Assoc J. 1999. PMID: 10028620
-
Bioaerosol Exposures and Respiratory Diseases in Cannabis Workers.Curr Allergy Asthma Rep. 2024 Jul;24(7):395-406. doi: 10.1007/s11882-024-01157-7. Epub 2024 Jun 15. Curr Allergy Asthma Rep. 2024. PMID: 38878249 Free PMC article. Review.
-
Lessons from Dairy Farmers for Occupational Allergy and Respiratory Disease.Curr Allergy Asthma Rep. 2023 Jun;23(6):325-339. doi: 10.1007/s11882-023-01081-2. Epub 2023 May 16. Curr Allergy Asthma Rep. 2023. PMID: 37191901 Free PMC article. Review.
Cited by
-
Draft Genome Sequence of Saccharopolyspora rectivirgula.Genome Announc. 2014 Jan 9;2(1):e01117-13. doi: 10.1128/genomeA.01117-13. Genome Announc. 2014. PMID: 24407634 Free PMC article.
-
Field sampling of indoor bioaerosols.Aerosol Sci Technol. 2020;54(5):572-584. doi: 10.1080/02786826.2019.1688759. Epub 2019 Nov 21. Aerosol Sci Technol. 2020. PMID: 31777412 Free PMC article.
-
Non-culturable bioaerosols in indoor settings: Impact on health and molecular approaches for detection.Atmos Environ (1994). 2015 Jun;110:45-53. doi: 10.1016/j.atmosenv.2015.03.039. Epub 2015 Mar 20. Atmos Environ (1994). 2015. PMID: 32288547 Free PMC article.
-
Presence of Archaea in the Indoor Environment and Their Relationships with Housing Characteristics.Microb Ecol. 2016 Aug;72(2):305-12. doi: 10.1007/s00248-016-0767-z. Epub 2016 Apr 20. Microb Ecol. 2016. PMID: 27098176
-
Recovery of Fungal Cells from Air Samples: a Tale of Loss and Gain.Appl Environ Microbiol. 2019 Apr 18;85(9):e02941-18. doi: 10.1128/AEM.02941-18. Print 2019 May 1. Appl Environ Microbiol. 2019. PMID: 30824432 Free PMC article.
References
-
- ACGIH 1999. Particle size-selective sampling for health-related aerosols. American Conference of Governmental Industrial Hygienists, Cincinnati, OH
-
- ATS 1998. Respiratory health hazards in agriculture. American Thoracic Society, Medical Section of the American Lung Association, New York, NY
-
- Bach HJ, Hartmann A, Schloter M, Munch JC. 2001. PCR primers and functional probes for amplification and detection of bacterial genes for extracellular peptidases in single strains and in soil. J. Microbiol. Methods 44:173–182 - PubMed
-
- Baker GC, Smith JJ, Cowan DA. 2003. Review and re-analysis of domain-specific 16S primers. J. Microbiol. Methods 55:541–555 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

