Preclinical pharmacology of novel indolecarboxamide ML-970, an investigative anticancer agent
- PMID: 22367116
- PMCID: PMC6690187
- DOI: 10.1007/s00280-012-1851-9
Preclinical pharmacology of novel indolecarboxamide ML-970, an investigative anticancer agent
Abstract
Purpose: ML-970 (AS-I-145; NSC 716970) is an indolecarboxamide synthesized as a less toxic analog of CC-1065 and duocarmycin, a natural product that binds the A-T-rich DNA minor groove and alkylates DNA. The NCI60 screening showed that ML-970 had potent cytotoxic activity, with an average GI(50) of 34 nM. The aim of this study is to define the pharmacological properties of this novel anticancer agent.
Methods: We established an HPLC method for the compound, examined its stability, protein binding, and metabolism by S9 enzymes, and conducted pharmacokinetic studies of the compound in two strains of mice using two different formulations.
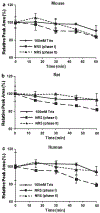
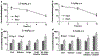
Results: ML-970 was relatively stable in plasma, being largely intact after an 8-h incubation in mouse plasma at 37°C. The compound was extensively bound to plasma proteins. ML-970 was only minimally metabolized by the enzymes present in S9 preparation and was not appreciably excreted in the urine or feces. The solution formulation provided higher C(max), AUC, F values, and greater bioavailability, although the suspension formulation resulted in a later T(max) and a slightly longer T(1/2). To determine the fate of the compound, we accomplished in-depth studies of tissue distribution; the results indicated that the compound undergoes extensive enterohepatic circulation.
Conclusions: The results obtained from this study will be relevant to the further development of the compound and may explain the lower myelotoxicity of this analog compared to CC-1065.
Figures


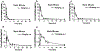

References
-
- Sato A, McNulty L, Cox K, Kim S, Scott A, Daniell K, Summerville K, Price C, Hudson S, Kiakos K, Hartley JA, Asao T, Lee M (2005) A novel class of in vivo active anticancer agents: achiral seco-amino- and seco-hydroxycyclopropylbenz[e]indolone (seco-CBI) analogues of the duocarmycins and CC-1065. J Med Chem 48:3903–3918 - PubMed
-
- Toth JL, Trzupek JD, Flores LV, Kiakos K, Hartley JA, Pennington WT, Lee M (2005) A novel achiral seco-amino-cyclopropylindoline (CI) analog of CC-1065 and the duocarmycins: design, synthesis and biological studies. Med Chem 1:13–19 - PubMed
-
- Kiakos K, Sato A, Asao T, McHugh PJ, Lee M, Hartley JA (2007) DNA sequence selective adenine alkylation, mechanism of adduct repair, and in vivo antitumor activity of the novel achiral seco-amino-cyclopropylbenz[e]indolone analogue of duocarmycin AS-I-145. Mol Cancer Ther 6:2708–2718 - PubMed
-
- Agrawal S, Zhang X, Cai Q, Kandimalla ER, Manning A, Jiang Z, Marcel T, Zhang R (1998) Effect of aspirin on protein binding and tissue disposition of oligonucleotide phosphorothioate in rats. J Drug Target 5:303–312 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

