Serum-soluble TRAIL levels in patients with severe persistent allergic asthma: its relation to omalizumab treatment
- PMID: 22367138
- PMCID: PMC3560751
- DOI: 10.12659/msm.882504
Serum-soluble TRAIL levels in patients with severe persistent allergic asthma: its relation to omalizumab treatment
Abstract
Background: In this study we compare the Omalizumab treatment modality in the dynamics of cell apoptosis regulating molecules in both severe persistent asthma patients who had no other any allergic disease, newly diagnosed patients with allergic asthma, and healthy volunteers.
Material/methods: Severe persistent allergic asthma patients were subjected to measurement of serum soluble TRAIL (TNF-related apoptosis-inducing ligand) levels during the active disease phase and the stable phase which occurred 4 months after Omalizumab treatment. Serum sTRAIL concentrations were measured by a solid phase sandwich enzyme-linked immunosorbent assay. Concentration levels were compared with those of age- and sex-matched newly diagnosed patients with allergic asthma, and healthy controls. All assays were carried out in duplicate. Total serum IgE levels, antinuclear antibody (ANA), rheumatoid factor (RF), hepatitis markers, C3, C4 and eosinophil levels were evaluated in all patients.
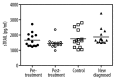
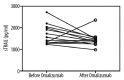
Results: ANA, RF, hepatitis markers were negative in all patients. Complement 3 and 4 levels were normal in all patients. Prick tests in all patients were detected in mite and grass allergy. These results correlated with specific IgE. There were no differences between the healthy controls, newly diagnosed allergic asthma patients, and non-treated severe persistent allergic asthma patients during the active phase. Interestingly, the levels in variances of the patients who had the effective omalizumab treatment were significantly lower than the healthy controls, while the mean values were not statistically significant.
Conclusions: Our study gives a different perspective on severe persistent allergic asthma and omalizumab treatment efficacy at the cell apoptosis-linked step by the serum sTRAIL levels.
Figures
References
-
- GINA (The Global Initiative For Asthma) 2009. p. 69.
-
- Yalcin AD, Oncel S, Akcan A, et al. Prevalance of allergic asthma, rhinitis and conjunctivitis in over 16 year old individuals in Antalya. Turkiye Klinikleri J Med Sci. 2010;30(3):888–94.
-
- National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma: expert panel report 2. Bethesda, Md: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Heart, Lung, and Blood Institute; 1997. NIH publication no. 97-4051.
-
- National Asthma Education and Prevention Program. Expert panel report: guidelines for the diagnosis and management of asthma update on selected topics - 2002. Bethesda, Md: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Heart, Lung, and Blood Institute; 2003. NIH publication no. 02-5074.
-
- Kumar V, Abbas AK, Nelson F, Aster J. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Saunders; 2010. p. 688.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

