Genetic dissection of pyrimidine biosynthesis and salvage in Leishmania donovani
- PMID: 22367196
- PMCID: PMC3339959
- DOI: 10.1074/jbc.M112.346502
Genetic dissection of pyrimidine biosynthesis and salvage in Leishmania donovani
Abstract
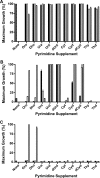
Protozoan parasites of the Leishmania genus express the metabolic machinery to synthesize pyrimidine nucleotides via both de novo and salvage pathways. To evaluate the relative contributions of pyrimidine biosynthesis and salvage to pyrimidine homeostasis in both life cycle stages of Leishmania donovani, individual mutant lines deficient in either carbamoyl phosphate synthetase (CPS), the first enzyme in pyrimidine biosynthesis, uracil phosphoribosyltransferase (UPRT), a salvage enzyme, or both CPS and UPRT were constructed. The Δcps lesion conferred pyrimidine auxotrophy and a growth requirement for medium supplementation with one of a plethora of pyrimidine nucleosides or nucleobases, although only dihydroorotate or orotate could circumvent the pyrimidine auxotrophy of the Δcps/Δuprt double knockout. The Δuprt null mutant was prototrophic for pyrimidines but could not salvage uracil or any pyrimidine nucleoside. The capability of the Δcps parasites to infect mice was somewhat diminished but still robust, indicating active pyrimidine salvage by the amastigote form of the parasite, but the Δcps/Δuprt mutant was completely attenuated with no persistent parasites detected after a 4-week infection. Complementation of the Δcps/Δuprt clone with either CPS or UPRT restored infectivity. These data establish that an intact pyrimidine biosynthesis pathway is essential for the growth of the promastigote form of L. donovani in culture, that all uracil and pyrimidine nucleoside salvage in the parasite is mediated by UPRT, and that both the biosynthetic and salvage pathways contribute to a robust infection of the mammalian host by the amastigote. These findings impact potential therapeutic design and vaccine strategies for visceral leishmaniasis.
Figures


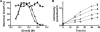
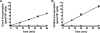

References
-
- Carter N., Rager N, Ullman B. (2003) Purine and pyrimidine transport and metabolism. in Molecular and Medical Parasitology (Marr J. J., Nilsen T. W., Komuniecki R., eds) pp. 197–223, Academic Press Ltd., London
-
- Hammond D. J., Gutteridge W. E. (1980) Enzymes of pyrimidine biosynthesis in Trypanosoma cruzi. FEBS Lett. 118, 259–262 - PubMed
-
- Nara T., Gao G., Yamasaki H., Nakajima-Shimada J., Aoki T. (1998) Carbamoyl-phosphate synthetase II in kinetoplastids. Biochim. Biophys. Acta 1387, 462–468 - PubMed
-
- Aronow B., Kaur K., McCartan K., Ullman B. (1987) Two high affinity nucleoside transporters in Leishmania donovani. Mol. Biochem. Parasitol. 22, 29–37 - PubMed
-
- Papageorgiou I. G., Yakob L., Al Salabi M. I., Diallinas G., Soteriadou K. P., De Koning H. P. (2005) Identification of the first pyrimidine nucleobase transporter in Leishmania. Similarities with the Trypanosoma brucei U1 transporter and antileishmanial activity of uracil analogues. Parasitology 130, 275–283 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

