A novel cardiomyocyte-enriched microRNA, miR-378, targets insulin-like growth factor 1 receptor: implications in postnatal cardiac remodeling and cell survival
- PMID: 22367207
- PMCID: PMC3339988
- DOI: 10.1074/jbc.M111.331751
A novel cardiomyocyte-enriched microRNA, miR-378, targets insulin-like growth factor 1 receptor: implications in postnatal cardiac remodeling and cell survival
Abstract
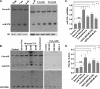
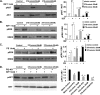
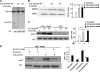
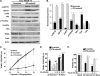
Postnatal cardiac remodeling is characterized by a marked decrease in the insulin-like growth factor 1 (IGF1) and IGF1 receptor (IGF1R) expression. The underlying mechanism remains unexplored. This study examined the role of microRNAs in postnatal cardiac remodeling. By expression profiling, we observed a 10-fold increase in miR-378 expression in 1-week-old neonatal mouse hearts compared with 16-day-old fetal hearts. There was also a 4-6-fold induction in expression of miR-378 in older (10 months) compared with younger (1 month) hearts. Interestingly, tissue distribution analysis identified miR-378 to be highly abundant in heart and skeletal muscles. In the heart, specific expression was observed in cardiac myocytes, which was inducible by a variety of stressors. Overexpression of miR-378 enhanced apoptosis of cardiomyocytes by direct targeting of IGF1R and reduced signaling in Akt cascade. The inhibition of miR-378 by its anti-miR protected cardiomyocytes against H(2)O(2) and hypoxia reoxygenation-induced cell death by promoting IGF1R expression and downstream Akt signaling cascade. Additionally, our data show that miR-378 expression is inhibited by IGF1 in cardiomyocytes. In tissues such as fibroblasts and fetal hearts, where IGF1 levels are high, we found either absent or significantly low miR-378 levels, suggesting an inverse relationship between these two factors. Our study identifies miR-378 as a new cardioabundant microRNA that targets IGF1R. We also demonstrate the existence of a negative feedback loop between miR-378, IGF1R, and IGF1 that is associated with postnatal cardiac remodeling and with the regulation of cardiomyocyte survival during stress.
Figures





References
-
- Lewis B. P., Burge C. B., Bartel D. P. (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 - PubMed
-
- Berezikov E., Guryev V., van de Belt J., Wienholds E., Plasterk R. H., Cuppen E. (2005) Phylogenetic shadowing and computational identification of human microRNA genes. Cell 120, 21–24 - PubMed
-
- Chen J. F., Murchison E. P., Tang R., Callis T. E., Tatsuguchi M., Deng Z., Rojas M., Hammond S. M., Schneider M. D., Selzman C. H., Meissner G., Patterson C., Hannon G. J., Wang D. Z. (2008) Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc. Natl. Acad. Sci. U.S.A. 105, 2111–2116 - PMC - PubMed
-
- da Costa Martins P. A., Bourajjaj M., Gladka M., Kortland M., van Oort R. J., Pinto Y. M., Molkentin J. D., De Windt L. J. (2008) Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation 118, 1567–1576 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

