Lung endothelial ADAM17 regulates the acute inflammatory response to lipopolysaccharide
- PMID: 22367719
- PMCID: PMC3403298
- DOI: 10.1002/emmm.201200217
Lung endothelial ADAM17 regulates the acute inflammatory response to lipopolysaccharide
Abstract
Acute lung injury (ALI) is associated with increased vascular permeability, leukocyte recruitment, and pro-inflammatory mediator release. We investigated the role of the metalloproteinase ADAM17 in endotoxin-induced ALI with focus on endothelial ADAM17. In vitro, endotoxin-mediated induction of endothelial permeability and IL-8-induced transmigration of neutrophils through human microvascular endothelial cells required ADAM17 as shown by inhibition with GW280264X or shRNA-mediated knockdown. In vivo, ALI was induced by intranasal endotoxin-challenge combined with GW280264X treatment or endothelial adam17-knockout. Endotoxin-triggered upregulation of ADAM17 mRNA in the lung was abrogated in knockout mice and associated with reduced ectodomain shedding of the junctional adhesion molecule JAM-A and the transmembrane chemokine CX3CL1. Induced vascular permeability, oedema formation, release of TNF-α and IL-6 and pulmonary leukocyte recruitment were all markedly reduced by GW280264X or endothelial adam17-knockout. Intranasal application of TNF-α could not restore leukocyte recruitment and oedema formation in endothelial adam17-knockout animals. Thus, activation of endothelial ADAM17 promotes acute pulmonary inflammation in response to endotoxin by multiple endothelial shedding events most likely independently of endothelial TNF-α release leading to enhanced vascular permeability and leukocyte recruitment.
Copyright © 2012 EMBO Molecular Medicine.
Figures

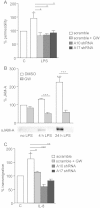
Transduced HMVEC-L were grown in transwell inserts and stimulated for 24 h with LPS (1 µg/ml) or were left unstimulated (PBS) in the presence or absence of GW280264X (10 µM). Permeability was measured by TRITC-dextran diffusion. Data are shown as percentage of TRITC-dextran permeability in relation to the unstimulated DMSO-treated control (= 100%).
HMVEC-L were stimulated for 4 or 24 h with LPS (1 µg/ml) or were left unstimulated (PBS) in the presence of 100 ng/ml LBP and in the absence of serum. Cells were treated either with GW280264X (10 µM) or with vehicle control (0.1% DMSO). Conditioned media were analysed by immunoblotting followed by densitometric quantification. A representative immunoblot of three independent experiments is shown below the graph.
Transduced HMVEC-L were pre-treated with or without GW280264X (10 µM) for 1.5 h and examined for IL-8-induced (10 ng/ml) transmigration of neutrophils. Experiments were performed with neutrophils from three different donors. Results were expressed as percentage of transmigration in relation to the LV-scramble-transduced control (C) receiving no IL-8.




Similar articles
-
Leukocytes require ADAM10 but not ADAM17 for their migration and inflammatory recruitment into the alveolar space.Blood. 2014 Jun 26;123(26):4077-88. doi: 10.1182/blood-2013-09-511543. Epub 2014 May 15. Blood. 2014. PMID: 24833351
-
Leukocyte ADAM17 regulates acute pulmonary inflammation.PLoS One. 2011;6(5):e19938. doi: 10.1371/journal.pone.0019938. Epub 2011 May 16. PLoS One. 2011. PMID: 21603616 Free PMC article.
-
Smooth muscle cells relay acute pulmonary inflammation via distinct ADAM17/ErbB axes.J Immunol. 2014 Jan 15;192(2):722-31. doi: 10.4049/jimmunol.1302496. Epub 2013 Dec 16. J Immunol. 2014. PMID: 24342803
-
Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules.Comb Chem High Throughput Screen. 2005 Mar;8(2):161-71. doi: 10.2174/1386207053258488. Comb Chem High Throughput Screen. 2005. PMID: 15777180 Review.
-
The role of ADAM-mediated shedding in vascular biology.Eur J Cell Biol. 2012 Jun-Jul;91(6-7):472-85. doi: 10.1016/j.ejcb.2011.09.003. Epub 2011 Dec 3. Eur J Cell Biol. 2012. PMID: 22138087 Review.
Cited by
-
Modulation of inflammatory responses by fractalkine signaling in microglia.PLoS One. 2021 May 21;16(5):e0252118. doi: 10.1371/journal.pone.0252118. eCollection 2021. PLoS One. 2021. PMID: 34019594 Free PMC article.
-
The Roles of Junctional Adhesion Molecules (JAMs) in Cell Migration.Front Cell Dev Biol. 2022 Mar 9;10:843671. doi: 10.3389/fcell.2022.843671. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35356274 Free PMC article. Review.
-
A Novel Experimental Approach for In Vivo Analyses of the Salivary Gland Microvasculature.Front Immunol. 2021 Feb 17;11:604470. doi: 10.3389/fimmu.2020.604470. eCollection 2020. Front Immunol. 2021. PMID: 33679695 Free PMC article.
-
The collectrin-like part of the SARS-CoV-1 and -2 receptor ACE2 is shed by the metalloproteinases ADAM10 and ADAM17.FASEB J. 2022 Mar;36(3):e22234. doi: 10.1096/fj.202101521R. FASEB J. 2022. PMID: 35199397 Free PMC article.
-
Secretome and Tunneling Nanotubes: A Multilevel Network for Long Range Intercellular Communication between Endothelial Cells and Distant Cells.Int J Mol Sci. 2021 Jul 26;22(15):7971. doi: 10.3390/ijms22157971. Int J Mol Sci. 2021. PMID: 34360735 Free PMC article. Review.
References
-
- Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, Hartmann D, Sedlacek R, Dietrich S, Muetze B, et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. 2004;172:6362–6372. - PubMed
-
- Alm AS, Li K, Chen H, Wang D, Andersson R, Wang X. Variation of lipopolysaccharide-induced acute lung injury in eight strains of mice. Respir Physiol Neurobiol. 2010;171:157–164. - PubMed
-
- Armstrong L, Godinho SI, Uppington KM, Whittington HA, Millar AB. Contribution of TNF-alpha converting enzyme and proteinase-3 to TNF-alpha processing in human alveolar macrophages. Am J Respir Cell Mol Biol. 2006;34:219–225. - PubMed
-
- Bzowska M, Jura N, Lassak A, Black RA, Bereta J. Tumour necrosis factor-alpha stimulates expression of TNF-alpha converting enzyme in endothelial cells. Eur J Biochem. 2004;271:2808–2820. - PubMed
-
- Canault M, Peiretti F, Kopp F, Bonardo B, Bonzi MF, Coudeyre JC, Alessi MC, Juhan-Vague I, Nalbone G. The TNF alpha converting enzyme (TACE/ADAM17) is expressed in the atherosclerotic lesions of apolipoprotein E-deficient mice: possible contribution to elevated plasma levels of soluble TNF alpha receptors. Atherosclerosis. 2006;187:82–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

