Inhibiting NF-κB in the developing lung disrupts angiogenesis and alveolarization
- PMID: 22367785
- PMCID: PMC3362264
- DOI: 10.1152/ajplung.00230.2011
Inhibiting NF-κB in the developing lung disrupts angiogenesis and alveolarization
Abstract
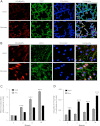
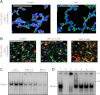
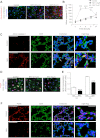
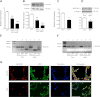
Bronchopulmonary dysplasia (BPD), a chronic lung disease of infancy, is characterized by arrested alveolar development. Pulmonary angiogenesis, mediated by the vascular endothelial growth factor (VEGF) pathway, is essential for alveolarization. However, the transcriptional regulators mediating pulmonary angiogenesis remain unknown. We previously demonstrated that NF-κB, a transcription factor traditionally associated with inflammation, plays a unique protective role in the neonatal lung. Therefore, we hypothesized that constitutive NF-κB activity is essential for postnatal lung development. Blocking NF-κB activity in 6-day-old neonatal mice induced the alveolar simplification similar to that observed in BPD and significantly reduced pulmonary capillary density. Studies to determine the mechanism responsible for this effect identified greater constitutive NF-κB in neonatal lung and in primary pulmonary endothelial cells (PEC) compared with adult. Moreover, inhibiting constitutive NF-κB activity in the neonatal PEC with either pharmacological inhibitors or RNA interference blocked PEC survival, decreased proliferation, and impaired in vitro angiogenesis. Finally, by chromatin immunoprecipitation, NF-κB was found to be a direct regulator of the angiogenic mediator, VEGF-receptor-2, in the neonatal pulmonary vasculature. Taken together, our data identify an entirely novel role for NF-κB in promoting physiological angiogenesis and alveolarization in the developing lung. Our data suggest that disruption of NF-κB signaling may contribute to the pathogenesis of BPD and that enhancement of NF-κB may represent a viable therapeutic strategy to promote lung growth and regeneration in pulmonary diseases marked by impaired angiogenesis.
Figures


Similar articles
-
Transforming Growth Factor-induced Protein Promotes NF-κB-mediated Angiogenesis during Postnatal Lung Development.Am J Respir Cell Mol Biol. 2021 Mar;64(3):318-330. doi: 10.1165/rcmb.2020-0153OC. Am J Respir Cell Mol Biol. 2021. PMID: 33264084 Free PMC article.
-
Endothelial-specific loss of IKKβ disrupts pulmonary endothelial angiogenesis and impairs postnatal lung growth.Am J Physiol Lung Cell Mol Physiol. 2023 Sep 1;325(3):L299-L313. doi: 10.1152/ajplung.00034.2023. Epub 2023 Jun 13. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 37310763 Free PMC article.
-
Activation of the nuclear factor-κB pathway during postnatal lung inflammation preserves alveolarization by suppressing macrophage inflammatory protein-2.Am J Physiol Lung Cell Mol Physiol. 2015 Sep 15;309(6):L593-604. doi: 10.1152/ajplung.00029.2015. Epub 2015 Jul 10. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26163511 Free PMC article.
-
Nuclear factor-kappa-B signaling in lung development and disease: one pathway, numerous functions.Birth Defects Res A Clin Mol Teratol. 2014 Mar;100(3):202-16. doi: 10.1002/bdra.23233. Epub 2014 Mar 17. Birth Defects Res A Clin Mol Teratol. 2014. PMID: 24639404 Free PMC article. Review.
-
Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease.Am J Respir Crit Care Med. 2007 May 15;175(10):978-85. doi: 10.1164/rccm.200611-1660PP. Epub 2007 Feb 1. Am J Respir Crit Care Med. 2007. PMID: 17272782 Free PMC article. Review.
Cited by
-
Developmental diversity and unique sensitivity to injury of lung endothelial subtypes during postnatal growth.iScience. 2023 Jan 31;26(3):106097. doi: 10.1016/j.isci.2023.106097. eCollection 2023 Mar 17. iScience. 2023. PMID: 36879800 Free PMC article.
-
Thiol-Redox Regulation in Lung Development and Vascular Remodeling.Antioxid Redox Signal. 2019 Oct 20;31(12):858-873. doi: 10.1089/ars.2018.7712. Epub 2019 Mar 4. Antioxid Redox Signal. 2019. PMID: 30648397 Free PMC article. Review.
-
Understanding the Impact of Infection, Inflammation, and Their Persistence in the Pathogenesis of Bronchopulmonary Dysplasia.Front Med (Lausanne). 2015 Dec 21;2:90. doi: 10.3389/fmed.2015.00090. eCollection 2015. Front Med (Lausanne). 2015. PMID: 26734611 Free PMC article. Review.
-
Emergent high fatality lung disease in systemic juvenile arthritis.Ann Rheum Dis. 2019 Dec;78(12):1722-1731. doi: 10.1136/annrheumdis-2019-216040. Epub 2019 Sep 27. Ann Rheum Dis. 2019. PMID: 31562126 Free PMC article.
-
Aberrant Pulmonary Vascular Growth and Remodeling in Bronchopulmonary Dysplasia.Front Med (Lausanne). 2016 May 20;3:21. doi: 10.3389/fmed.2016.00021. eCollection 2016. Front Med (Lausanne). 2016. PMID: 27243014 Free PMC article. Review.
References
-
- Ahlbrecht K, Schmitz J, Seay U, Schwarz C, Mittnacht-Kraus R, Gaumann A, Haberberger RV, Herold S, Breier G, Grimminger F, Seeger W, Voswinckel R. Spatiotemporal expression of flk-1 in pulmonary epithelial cells during lung development. Am J Respir Cell Mol Biol 39: 163–170, 2008 - PubMed
-
- Aoki T, Kataoka H, Ishibashi R, Nozaki K, Morishita R, Hashimoto N. Reduced collagen biosynthesis is the hallmark of cerebral aneurysm: contribution of interleukin-1beta and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol 29: 1080–1086, 2009 - PubMed
-
- Aoki T, Kataoka H, Shimamura M, Nakagami H, Wakayama K, Moriwaki T, Ishibashi R, Nozaki K, Morishita R, Hashimoto N. NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation 116: 2830–2840, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

