Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory
- PMID: 22368036
- PMCID: PMC3942786
- DOI: 10.1002/ana.23536
Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory
Abstract
Objective: There are approximately 8.5 million Alzheimer disease (AD) patients who need anesthesia and surgery care every year. The inhalation anesthetic isoflurane, but not desflurane, has been shown to induce caspase activation and apoptosis, which are part of AD neuropathogenesis, through the mitochondria-dependent apoptosis pathway. However, the in vivo relevance, underlying mechanisms, and functional consequences of these findings remain largely to be determined.
Methods: We therefore set out to assess the effects of isoflurane and desflurane on mitochondrial function, cytotoxicity, learning, and memory using flow cytometry, confocal microscopy, Western blot analysis, immunocytochemistry, and the fear conditioning test.
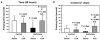
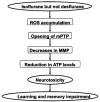
Results: Here we show that isoflurane, but not desflurane, induces opening of mitochondrial permeability transition pore (mPTP), increase in levels of reactive oxygen species, reduction in levels of mitochondrial membrane potential and adenosine-5'-triphosphate, activation of caspase 3, and impairment of learning and memory in cultured cells, mouse hippocampus neurons, mouse hippocampus, and mice. Moreover, cyclosporine A, a blocker of mPTP opening, attenuates isoflurane-induced mPTP opening, caspase 3 activation, and impairment of learning and memory. Finally, isoflurane may induce the opening of mPTP via increasing levels of reactive oxygen species.
Interpretation: These findings suggest that desflurane could be a safer anesthetic for AD patients as compared to isoflurane, and elucidate the potential mitochondria-associated underlying mechanisms, and therefore have implications for use of anesthetics in AD patients, pending human study confirmation.
Copyright © 2012 American Neurological Association.
Figures


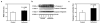




References
-
- Alzheimer’s Association . Alzheimer’s Disease Facts and Figures. Alzheimer’s Association; Chicago, IL: 2011. http://www.alz.org/downloads/Facts_Figures_2011.pdf. - PubMed
-
- Silbert B, Evered L, Scott DA, Maruff P. Anesthesiology must play a greater role in patients with Alzheimer’s disease. Anesth Analg. 2011;112:1242–1245. - PubMed
-
- Eckenhoff RG, Johansson JS, Wei H, et al. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

