Synergistic effects of central nervous system-directed gene therapy and bone marrow transplantation in the murine model of infantile neuronal ceroid lipofuscinosis
- PMID: 22368049
- PMCID: PMC3369009
- DOI: 10.1002/ana.23545
Synergistic effects of central nervous system-directed gene therapy and bone marrow transplantation in the murine model of infantile neuronal ceroid lipofuscinosis
Abstract
Objective: Infantile neuronal ceroid lipofuscinosis (INCL) is an inherited childhood neurodegenerative disorder caused by the loss of palmitoyl protein thioesterase-1 (PPT1) activity. Affected children suffer from blindness, epilepsy, motor dysfunction, cognitive decline, and premature death. The Ppt1(-/-) mouse shares the histological and clinical features of INCL. Previous single-therapy approaches using small molecule drugs, gene therapy, or neuronal stem cells resulted in partial histological correction, with minimal improvements in motor function or lifespan. Here, we combined central nervous system (CNS)-directed adeno-associated virus (AAV)2/5-mediated gene therapy with bone marrow transplantation (BMT) in the INCL mouse.
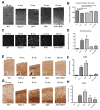
Methods: At birth, Ppt1(-/-) and wild-type mice were given either intracranial injections of AAV2/5-PPT1 or bone marrow transplantation, separately as well as in combination. To assess function, we measured rotorod performance monthly as well as lifespan. At terminal time points, we evaluated the therapeutic effects on several INCL-specific parameters, such as cortical thickness, autofluorescent accumulation, and glial activation. Finally, we determined levels of PPT1 enzyme activity and bone marrow engraftment in treated mice.
Results: AAV2/5-mediated gene therapy alone resulted in significant histological correction, improved motor function, and increased lifespan. Interestingly, the addition of BMT further increased the lifespan of treated mice and led to dramatic, sustained improvements in motor function. These data are truly striking, given that BMT alone is ineffective, yet it synergizes with CNS-directed gene therapy to dramatically increase efficacy and lifespan.
Interpretation: AAV2/5-mediated gene therapy in combination with BMT provides an unprecedented increase in lifespan as well as dramatic improvement on functional and histological parameters.
Copyright © 2012 American Neurological Association.
Figures
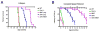
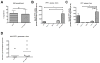
Similar articles
-
CNS-directed AAV2-mediated gene therapy ameliorates functional deficits in a murine model of infantile neuronal ceroid lipofuscinosis.Mol Ther. 2006 Mar;13(3):538-47. doi: 10.1016/j.ymthe.2005.11.008. Epub 2005 Dec 20. Mol Ther. 2006. PMID: 16364693
-
Combination small molecule PPT1 mimetic and CNS-directed gene therapy as a treatment for infantile neuronal ceroid lipofuscinosis.J Inherit Metab Dis. 2012 Sep;35(5):847-57. doi: 10.1007/s10545-011-9446-x. Epub 2012 Feb 7. J Inherit Metab Dis. 2012. PMID: 22310926 Free PMC article.
-
Synergistic effects of treating the spinal cord and brain in CLN1 disease.Proc Natl Acad Sci U S A. 2017 Jul 18;114(29):E5920-E5929. doi: 10.1073/pnas.1701832114. Epub 2017 Jul 3. Proc Natl Acad Sci U S A. 2017. PMID: 28673981 Free PMC article.
-
Pathogenesis and therapies for infantile neuronal ceroid lipofuscinosis (infantile CLN1 disease).Biochim Biophys Acta. 2013 Nov;1832(11):1906-9. doi: 10.1016/j.bbadis.2013.05.026. Epub 2013 Jun 6. Biochim Biophys Acta. 2013. PMID: 23747979 Free PMC article. Review.
-
CLN2 Disease (Classic Late Infantile Neuronal Ceroid Lipofuscinosis).Pediatr Endocrinol Rev. 2016 Jun;13 Suppl 1:682-8. Pediatr Endocrinol Rev. 2016. PMID: 27491216 Review.
Cited by
-
Oxidative stress as a therapeutic target in globoid cell leukodystrophy.Exp Neurol. 2012 Oct;237(2):444-52. doi: 10.1016/j.expneurol.2012.07.013. Epub 2012 Jul 28. Exp Neurol. 2012. PMID: 22849820 Free PMC article.
-
Neuronal Ceroid Lipofuscinosis: Potential for Targeted Therapy.Drugs. 2021 Jan;81(1):101-123. doi: 10.1007/s40265-020-01440-7. Drugs. 2021. PMID: 33242182 Review.
-
Glial cells are functionally impaired in juvenile neuronal ceroid lipofuscinosis and detrimental to neurons.Acta Neuropathol Commun. 2017 Oct 17;5(1):74. doi: 10.1186/s40478-017-0476-y. Acta Neuropathol Commun. 2017. PMID: 29041969 Free PMC article.
-
Current and Emerging Treatment Strategies for Neuronal Ceroid Lipofuscinoses.CNS Drugs. 2019 Apr;33(4):315-325. doi: 10.1007/s40263-019-00620-8. CNS Drugs. 2019. PMID: 30877620 Free PMC article. Review.
-
Reversible Cysteine Acylation Regulates the Activity of Human Palmitoyl-Protein Thioesterase 1 (PPT1).PLoS One. 2016 Jan 5;11(1):e0146466. doi: 10.1371/journal.pone.0146466. eCollection 2016. PLoS One. 2016. PMID: 26731412 Free PMC article.
References
-
- Hofmann SL, Peltonen L. The neuronal ceroid lipofuscinosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3877–3894.
-
- Bible E, Gupta P, Hofmann SL, et al. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2004;16:346–359. - PubMed
-
- Griffey M, Bible E, Vogler C, et al. Adeno-associated virus 2-mediated gene therapy decreases autofluorescent storage material and increases brain mass in a murine model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2004;16:360–369. - PubMed
-
- Griffey MA, Wozniak D, Wong M, et al. CNS-directed AAV2-mediated gene therapy ameliorates functional deficits in a murine model of infantile neuronal ceroid lipofuscinosis. Mol Ther. 2006;13:538–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

