Borders and comparative cytoarchitecture of the perirhinal and postrhinal cortices in an F1 hybrid mouse
- PMID: 22368084
- PMCID: PMC3584955
- DOI: 10.1093/cercor/bhs038
Borders and comparative cytoarchitecture of the perirhinal and postrhinal cortices in an F1 hybrid mouse
Abstract
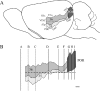
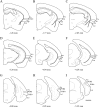
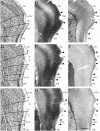
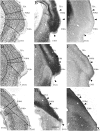
We examined the cytoarchitectonic and chemoarchitectonic organization of the cortical regions associated with the posterior rhinal fissure in the mouse brain, within the framework of what is known about these regions in the rat. Primary observations were in a first-generation hybrid mouse line, B6129PF/J1. The F1 hybrid was chosen because of the many advantages afforded in the study of the molecular and cellular bases of learning and memory. Comparisons with the parent strains, the C57BL6/J and 129P3/J are also reported. Mouse brain tissue was processed for visualization of Nissl material, myelin, acetyl cholinesterase, parvalbumin, and heavy metals. Tissue stained for heavy metals by the Timm's method was particularly useful in the assignment of borders and in the comparative analyses because the patterns of staining were similar across species and strains. As in the rat, the areas examined were parcellated into 2 regions, the perirhinal and the postrhinal cortices. The perirhinal cortex was divided into areas 35 and 36, and the postrhinal cortex was divided into dorsal (PORd) and ventral (PORv) subregions. In addition to identifying the borders of the perirhinal cortex, we were able to identify a region in the mouse brain that shares signature features with the rat postrhinal cortex.
Figures










References
-
- Aggleton JP, Brown MW. Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Q J Exp Psychol B. 2005;58:218–233. - PubMed
-
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. J CompNeurol. 1987;264:326–355. - PubMed
-
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig (Germany): Barth; 1909.
-
- Bucci DJ, Burwell RD. Deficits in attentional orienting following damage to the perirhinal or postrhinal cortices. Behav Neurosci. 2004;118:1117–1122. - PubMed
-
- Buckley MJ, Gaffan D. Perirhinal cortical contributions to object perception. Trends Cogn Sci. 2006;10:100–107. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

