Management of tetralogy of fallot with pulmonary atresia
- PMID: 22368654
- PMCID: PMC3232551
Management of tetralogy of fallot with pulmonary atresia
Abstract
Tetralogy of Fallot with Pulmonary Atresia is an extreme form of tetralogy characterized by absence of flow from the right ventricle to the pulmonary arteries. Pulmonary blood flow is derived from a variety of sources, including native pulmonary artery branches and aorto-pulmonary collaterals with significant variability from patient to patient. Management must therefore be individualized to each patient's anatomy and physiology. Cardiac catheterization plays a crucial diagnostic and therapeutic role in this group of patients. This article is a concise review of the spectrum of anatomic variability seen in this lesion with an emphasis on diagnostic and therapeutic catheterization . It also highlights our staged surgical approach to this lesion and provides data on long-term outcome after complete intracardiac repair.
Keywords: Pulmonary Atresia; Tetralogy of Fallot.
Figures




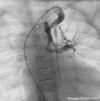


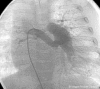

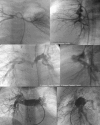





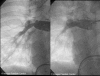
References
-
- Thiene G, Frescura C, Bini RM, Valente M, Gallucci V. Histology of pulmonary arterial supply in pulmonary atresia with ventricular septal defect. Circulation. 1979;60:1066–1074. - PubMed
-
- Iyer KS, Mee RB. Staged repair of pulmonary atresia with ventricular septal defect and major systemic to pulmonary artery collaterals. Ann Thorac Surg. 1991;51:65–72. - PubMed
LinkOut - more resources
Full Text Sources
