Characterizing the role of rice NRAMP5 in Manganese, Iron and Cadmium Transport
- PMID: 22368778
- PMCID: PMC3285952
- DOI: 10.1038/srep00286
Characterizing the role of rice NRAMP5 in Manganese, Iron and Cadmium Transport
Abstract
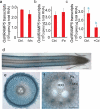
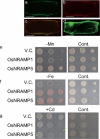
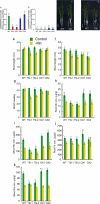
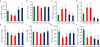
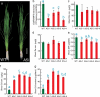
Metals like manganese (Mn) and iron (Fe) are essential for metabolism, while cadmium (Cd) is toxic for virtually all living organisms. Understanding the transport of these metals is important for breeding better crops. We have identified that OsNRAMP5 contributes to Mn, Fe and Cd transport in rice. OsNRAMP5 expression was restricted to roots epidermis, exodermis, and outer layers of the cortex as well as in tissues around the xylem. OsNRAMP5 localized to the plasma membrane, and complemented the growth of yeast strains defective in Mn, Fe, and Cd transport. OsNRAMP5 RNAi (OsNRAMP5i) plants accumulated less Mn in the roots, and less Mn and Fe in shoots, and xylem sap. The suppression of OsNRAMP5 promoted Cd translocation to shoots, highlighting the importance of this gene for Cd phytoremediation. These data reveal that OsNRAMP5 contributes to Mn, Cd, and Fe transport in rice and is important for plant growth and development.
Figures
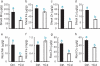
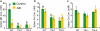
Similar articles
-
OsNRAMP5, a major player for constitutive iron and manganese uptake in rice.Plant Signal Behav. 2012 Jul;7(7):763-6. doi: 10.4161/psb.20510. Epub 2012 Jul 1. Plant Signal Behav. 2012. PMID: 22751306 Free PMC article.
-
OsNRAMP5 contributes to manganese translocation and distribution in rice shoots.J Exp Bot. 2014 Sep;65(17):4849-61. doi: 10.1093/jxb/eru259. Epub 2014 Jun 24. J Exp Bot. 2014. PMID: 24963001 Free PMC article.
-
Overexpression of the manganese/cadmium transporter OsNRAMP5 reduces cadmium accumulation in rice grain.J Exp Bot. 2020 Sep 19;71(18):5705-5715. doi: 10.1093/jxb/eraa287. J Exp Bot. 2020. PMID: 32542348
-
Toxicometallomics of Cadmium, Manganese and Arsenic with Special Reference to the Roles of Metal Transporters.Toxicol Res. 2019 Oct;35(4):311-317. doi: 10.5487/TR.2019.35.4.311. Epub 2019 Oct 15. Toxicol Res. 2019. PMID: 31636842 Free PMC article. Review.
-
Sustainable management of cadmium-contaminated soils as affected by exogenous application of nutrients: A review.J Environ Manage. 2021 Oct 1;295:113081. doi: 10.1016/j.jenvman.2021.113081. Epub 2021 Jun 23. J Environ Manage. 2021. PMID: 34171783 Review.
Cited by
-
Citric Acid Inhibits Cd Absorption and Transportation by Improving the Antagonism of Essential Elements in Rice Organs.Toxics. 2024 Jun 14;12(6):431. doi: 10.3390/toxics12060431. Toxics. 2024. PMID: 38922111 Free PMC article.
-
The expression of heterologous Fe (III) phytosiderophore transporter HvYS1 in rice increases Fe uptake, translocation and seed loading and excludes heavy metals by selective Fe transport.Plant Biotechnol J. 2017 Apr;15(4):423-432. doi: 10.1111/pbi.12637. Epub 2016 Oct 10. Plant Biotechnol J. 2017. PMID: 27633505 Free PMC article.
-
AhNRAMP1 iron transporter is involved in iron acquisition in peanut.J Exp Bot. 2012 Jul;63(12):4437-46. doi: 10.1093/jxb/ers117. Epub 2012 May 18. J Exp Bot. 2012. PMID: 22611231 Free PMC article.
-
Characterizing the crucial components of iron homeostasis in the maize mutants ys1 and ys3.PLoS One. 2013 May 8;8(5):e62567. doi: 10.1371/journal.pone.0062567. Print 2013. PLoS One. 2013. PMID: 23667491 Free PMC article.
-
Soil applied silicon and manganese combined with foliar application of 5-aminolevulinic acid mediate photosynthetic recovery in Cd-stressed Salvia miltiorrhiza by regulating Cd-transporter genes.Front Plant Sci. 2022 Sep 29;13:1011872. doi: 10.3389/fpls.2022.1011872. eCollection 2022. Front Plant Sci. 2022. PMID: 36247621 Free PMC article.
References
-
- Marschner H. Mineral nutrition of higher plants. 2nd edn, (Academic Press, 1995).
-
- Alscher R. G., Erturk N. & Heath L. S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 53, 1331–1341 (2002). - PubMed
-
- Britt R. D. Oxygen evolution. in Oxygenic photosynthesis: the light reactions. Vol. Advances in Photosynthesis and Respiration. 4, 137–159 (Kluwer Academic Publishers 1996).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

