A comparative structural bioinformatics analysis of inherited mutations in β-D-Mannosidase across multiple species reveals a genotype-phenotype correlation
- PMID: 22369051
- PMCID: PMC3333182
- DOI: 10.1186/1471-2164-12-S3-S22
A comparative structural bioinformatics analysis of inherited mutations in β-D-Mannosidase across multiple species reveals a genotype-phenotype correlation
Abstract
Background: Lysosomal β-D-mannosidase is a glycosyl hydrolase that breaks down the glycosidic bonds at the non-reducing end of N-linked glycoproteins. Hence, it is a crucial enzyme in polysaccharide degradation pathway. Mutations in the MANBA gene that codes for lysosomal β-mannosidase, result in improper coding and malfunctioning of protein, leading to β-mannosidosis. Studying the location of mutations on the enzyme structure is a rational approach in order to understand the functional consequences of these mutations. Accordingly, the pathology and clinical manifestations of the disease could be correlated to the genotypic modifications.
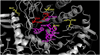
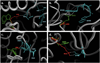
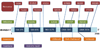
Results: The wild-type and inherited mutations of β-mannosidase were studied across four different species, human, cow, goat and mouse employing a previously demonstrated comprehensive homology modeling and mutational mapping technique, which reveals a correlation between the variation of genotype and the severity of phenotype in β-mannosidosis. X-ray crystallographic structure of β-mannosidase from Bacteroides thetaiotaomicron was used as template for 3D structural modeling of the wild-type enzymes containing all the associated ligands. These wild-type models subsequently served as templates for building mutational structures. Truncations account for approximately 70% of the mutational cases. In general, the proximity of mutations to the active site determines the severity of phenotypic expressions. Mapping mutations to the MANBA gene sequence has identified five mutational hot-spots.
Conclusion: Although restrained by a limited dataset, our comprehensive study suggests a genotype-phenotype correlation in β-mannosidosis. A predictive approach for detecting likely β-mannosidosis is also demonstrated where we have extrapolated observed mutations from one species to homologous positions in other organisms based on the proximity of the mutations to the enzyme active site and their co-location from different organisms. Apart from aiding the detection of mutational hotspots in the gene, where novel mutations could be disease-implicated, this approach also provides a way to predict new disease mutations. Higher expression of the exoglycosidase chitobiase is said to play a vital role in determining disease phenotypes in human and mouse. A bigger dataset of inherited mutations as well as a parallel study of β-mannosidase and chitobiase activities in prospective patients would be interesting to better understand the underlying reasons for β-mannosidosis.
Figures




References
-
- Samra ZQ, Athar MA. Cloning, sequence, expression and characterization of human beta-mannosidase. Acta Biochim Pol. 2008;55(3):479–490. - PubMed
-
- Seal RL, Gordon SM, Lush MJ, Wright MW, Bruford EA. genenames.org: the HGNC resources in 2011. Nucleic Acids Res. 2011;39(Database issue):D514–519. - PMC - PubMed
-
- Cooper A, Sardharwalla IB, Roberts MM. Human beta-mannosidase deficiency. The New England journal of medicine. 1986;315(19):1231. - PubMed
-
- Jones MZ, Dawson G. Caprine beta-mannosidosis. Inherited deficiency of beta-D-mannosidase. J Biol Chem. 1981;256(10):5185–5188. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

