OccK channels from Pseudomonas aeruginosa exhibit diverse single-channel electrical signatures but conserved anion selectivity
- PMID: 22369314
- PMCID: PMC3311111
- DOI: 10.1021/bi300066w
OccK channels from Pseudomonas aeruginosa exhibit diverse single-channel electrical signatures but conserved anion selectivity
Abstract
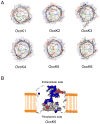
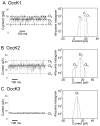
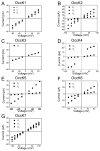
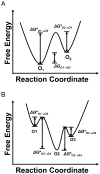
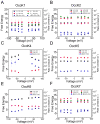
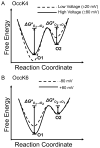
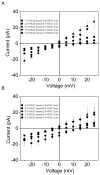
Pseudomonas aeruginosa is a Gram-negative bacterium that utilizes substrate-specific outer membrane (OM) proteins for the uptake of small, water-soluble nutrients employed in the growth and function of the cell. In this paper, we present for the first time a comprehensive single-channel examination of seven members of the OM carboxylate channel K (OccK) subfamily. Recent biochemical, functional, and structural characterization of the OccK proteins revealed their common features, such as a closely related, monomeric, 18-stranded β-barrel conformation with a kidney-shaped transmembrane pore and the presence of a basic ladder within the channel lumen. Here, we report that the OccK proteins exhibited fairly distinct unitary conductance values, in a much broader range than previously expected, which includes low (~40-100 pS) and medium (~100-380 pS) conductance. These proteins showed diverse single-channel dynamics of current gating transitions, revealing one-open substate (OccK3), two-open substate (OccK4-OccK6), and three-open substate (OccK1, OccK2, and OccK7) kinetics with functionally distinct conformations. Interestingly, we discovered that anion selectivity is a conserved trait among the members of the OccK subfamily, confirming the presence of a net pool of positively charged residues within their central constriction. Moreover, these results are in accord with an increased specificity and selectivity of these protein channels for negatively charged, carboxylate-containing substrates. Our findings might ignite future functional examinations and full atomistic computational studies for unraveling a mechanistic understanding of the passage of small molecules across the lumen of substrate-specific, β-barrel OM proteins.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

