BRCA1 tumor suppressor network: focusing on its tail
- PMID: 22369660
- PMCID: PMC3315748
- DOI: 10.1186/2045-3701-2-6
BRCA1 tumor suppressor network: focusing on its tail
Abstract
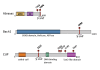
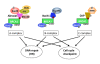
Germline mutations of the BRCA1 tumor suppressor gene are a major cause of familial breast and ovarian cancer. BRCA1 plays critical roles in the DNA damage response that regulates activities of multiple repair and checkpoint pathways for maintaining genome stability. The BRCT domains of BRCA1 constitute a phospho-peptide binding domain recognizing a phospho-SPxF motif (S, serine; P, proline; × varies; F, phenylalanine). The BRCT domains are frequently targeted by clinically important mutations and most of these mutations disrupt the binding surface of the BRCT domains to phosphorylated peptides. The BRCT domain and its capability to bind phosphorylated protein is required for the tumor suppressor function of BRCA1. Through its BRCT phospho-binding ability BRCA1 forms at least three mutually exclusive complexes by binding to phosphorylated proteins Abraxas, Bach1 and CTIP. The A, B and C complexes, at lease partially undertake BRCA1's role in mechanisms of cell cycle checkpoint and DNA repair that maintain genome stability, thus may play important roles in BRCA1's tumor suppressor function.
Figures

Similar articles
-
The BRCA1-interacting protein Abraxas is required for genomic stability and tumor suppression.Cell Rep. 2014 Aug 7;8(3):807-17. doi: 10.1016/j.celrep.2014.06.050. Epub 2014 Jul 24. Cell Rep. 2014. PMID: 25066119 Free PMC article.
-
Structure of BRCA1-BRCT/Abraxas Complex Reveals Phosphorylation-Dependent BRCT Dimerization at DNA Damage Sites.Mol Cell. 2016 Feb 4;61(3):434-448. doi: 10.1016/j.molcel.2015.12.017. Epub 2016 Jan 14. Mol Cell. 2016. PMID: 26778126 Free PMC article.
-
BRCA1 and Its Vulnerable C-Terminal BRCT Domain: Structure, Function, Genetic Mutations and Links to Diagnosis and Treatment of Breast and Ovarian Cancer.Pharmaceuticals (Basel). 2024 Mar 4;17(3):333. doi: 10.3390/ph17030333. Pharmaceuticals (Basel). 2024. PMID: 38543119 Free PMC article. Review.
-
Phosphopeptide binding specificities of BRCA1 COOH-terminal (BRCT) domains.J Biol Chem. 2003 Dec 26;278(52):52914-8. doi: 10.1074/jbc.C300407200. Epub 2003 Oct 24. J Biol Chem. 2003. PMID: 14578343
-
BRCA1 the Versatile Defender: Molecular to Environmental Perspectives.Int J Mol Sci. 2023 Sep 19;24(18):14276. doi: 10.3390/ijms241814276. Int J Mol Sci. 2023. PMID: 37762577 Free PMC article. Review.
Cited by
-
The BRCA1-interacting protein Abraxas is required for genomic stability and tumor suppression.Cell Rep. 2014 Aug 7;8(3):807-17. doi: 10.1016/j.celrep.2014.06.050. Epub 2014 Jul 24. Cell Rep. 2014. PMID: 25066119 Free PMC article.
-
RNF8- and Ube2S-Dependent Ubiquitin Lysine 11-Linkage Modification in Response to DNA Damage.Mol Cell. 2017 May 18;66(4):458-472.e5. doi: 10.1016/j.molcel.2017.04.013. Mol Cell. 2017. PMID: 28525740 Free PMC article.
-
ABRAXAS1 orchestrates BRCA1 activities to counter genome destabilizing repair pathways-lessons from breast cancer patients.Cell Death Dis. 2023 May 17;14(5):328. doi: 10.1038/s41419-023-05845-6. Cell Death Dis. 2023. PMID: 37198153 Free PMC article.
-
Brca1 deficiency causes bone marrow failure and spontaneous hematologic malignancies in mice.Blood. 2016 Jan 21;127(3):310-3. doi: 10.1182/blood-2015-03-635599. Epub 2015 Dec 7. Blood. 2016. PMID: 26644450 Free PMC article.
-
ATM orchestrates the DNA-damage response to counter toxic non-homologous end-joining at broken replication forks.Nat Commun. 2019 Jan 8;10(1):87. doi: 10.1038/s41467-018-07729-2. Nat Commun. 2019. PMID: 30622252 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

