PDGF mediates TGFβ-induced migration during development of the spinous process
- PMID: 22369999
- PMCID: PMC3322265
- DOI: 10.1016/j.ydbio.2012.02.013
PDGF mediates TGFβ-induced migration during development of the spinous process
Abstract
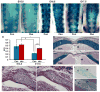
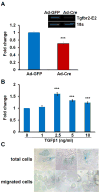
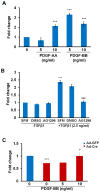
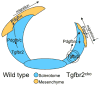
Mechanisms mediating closure of the dorsal vertebrae are not clear. Previously, we showed that deletion of TGFβ type II receptor (Tgfbr2) in sclerotome in mice results in failure in the formation of the spinous process, mimicking spina bifida occulta, a common malformation in humans. In this study, we aimed to determine whether missing dorsal structures in Tgfbr2 mutant mice were due to defects in mesenchymal migration and to clarify mechanism of TGFβ-mediated migration. First, we showed that gross alterations in dorsal vertebrae were apparent by E16.5days in Tgfbr2 mutants. In addition, histological staining showed that the mesenchyme adjacent to the developing cartilage was thin compared to controls likely due to reduced proliferation and migration of these cells. Next, we used a chemotaxis migration assay to show that TGFβ promotes migration in mixed cultures of embryonic sclerotome and associated mesenchyme. TGFβ stimulated expression of PDGF ligands and receptors in the cultures and intact PDGF signaling was required for TGFβ-mediated migration. Since PDGF ligands are expressed in the sclerotome-derived cartilage where Tgfbr2 is deleted and the receptors are predominantly expressed in the adjacent mesenchyme, we propose that TGFβ acts on the sclerotome to regulate expression of PDGF ligands, which then act on the associated mesenchyme in a paracrine fashion to mediate proliferation, migration and subsequent differentiation of the adjacent sclerotome.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Aase K, Abramsson A, Karlsson L, Betsholtz C, Eriksson U. Expression analysis of PDGF-C in adult and developing mouse tissues. Mechanisms of development. 2002;110:187–191. - PubMed
-
- Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R. Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol. 2004;276:124–142. - PubMed
-
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. - PubMed
-
- Bornfeldt KE, Raines EW, Graves LM, Skinner MP, Krebs EG, Ross R. Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Ann N Y Acad Sci. 1995;766:416–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

