Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987
- PMID: 22370319
- PMCID: PMC3341152
- DOI: 10.1200/JCO.2011.38.6979
Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987
Erratum in
-
Errata.J Clin Oncol. 2018 Mar 10;36(8):833. doi: 10.1200/JCO.2018.78.0759. J Clin Oncol. 2018. PMID: 31329711 Free PMC article.
Abstract
Purpose: The combination of gemcitabine plus cisplatin (GC) is a standard regimen in patients with locally advanced or metastatic urothelial cancer. A phase I/II study suggested that a three-drug regimen that included paclitaxel had greater antitumor activity and might improve survival.
Patients and methods: We conducted a randomized phase III study to compare paclitaxel/cisplatin/gemcitabine (PCG) with GC in patients with locally advanced or metastatic urothelial carcinoma. Primary outcome was overall survival (OS). Secondary outcomes were progression-free survival (PFS), overall response rate, and toxicity.
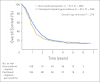
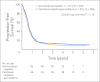
Results: From 2001 to 2004, 626 patients were randomly assigned; 312 patients were assigned to PCG, and 314 patients were assigned to GC. After a median follow-up of 4.6 years, the median OS was 15.8 months on PCG versus 12.7 months on GC (hazard ratio [HR], 0.85; P = .075). OS in the subgroup of all eligible patients was significantly longer on PCG (3.2 months; HR, 0.82; P = .03), as was the case in patients with bladder primary tumors. PFS was not significantly longer on PCG (HR, 0.87; P = .11). Overall response rate was 55.5% on PCG and 43.6% on GC (P = .0031). Both treatments were well tolerated, with more thrombocytopenia and bleeding on GC than PCG (11.4% v 6.8%, respectively; P = .05) and more febrile neutropenia on PCG than GC (13.2% v 4.3%, respectively; P < .001).
Conclusion: The addition of paclitaxel to GC provides a higher response rate and a 3.1-month survival benefit that did not reach statistical significance. Novel approaches will be required to obtain major improvements in survival of incurable urothelial cancer.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Sternberg CN, Yagoda A, Scher HI, et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium: Efficacy and patterns of response and relapse. Cancer. 1989;64:2448–2458. - PubMed
-
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. - PubMed
-
- Loehrer PJ, Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J Clin Oncol. 1992;10:1066–1073. - PubMed
-
- Logothetis CJ, Dexeus FH, Finn L, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol. 1990;8:1050–1055. - PubMed
-
- Bellmunt J, Ribas A, Eres N, et al. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer. 1997;80:1966–1972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2U10 CA11488-32/CA/NCI NIH HHS/United States
- 2U10 CA11488-33/CA/NCI NIH HHS/United States
- U10 CA011488/CA/NCI NIH HHS/United States
- 2U10 CA11488-39/CA/NCI NIH HHS/United States
- 2U10 CA11488-35/CA/NCI NIH HHS/United States
- MRC_/Medical Research Council/United Kingdom
- 2U10 CA11488-31/CA/NCI NIH HHS/United States
- 2U10 CA11488-34/CA/NCI NIH HHS/United States
- C448/A2683/CRUK_/Cancer Research UK/United Kingdom
- 2U10 CA11488-30/CA/NCI NIH HHS/United States
- 2U10 CA11488-36/CA/NCI NIH HHS/United States
- 2U10 CA11488-38/CA/NCI NIH HHS/United States
- 2U10 CA11488-37/CA/NCI NIH HHS/United States
- 2U10 CA11488-40/CA/NCI NIH HHS/United States
- 2U10 CA11488-41/CA/NCI NIH HHS/United States
- U10 CA077202/CA/NCI NIH HHS/United States
- CA077202/CA/NCI NIH HHS/United States
- 2U10 CA11488-29/CA/NCI NIH HHS/United States
- 2U10 CA11488-28/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

