Defects in gallbladder emptying and bile Acid homeostasis in mice with cystic fibrosis transmembrane conductance regulator deficiencies
- PMID: 22370478
- PMCID: PMC3579557
- DOI: 10.1053/j.gastro.2012.02.033
Defects in gallbladder emptying and bile Acid homeostasis in mice with cystic fibrosis transmembrane conductance regulator deficiencies
Abstract
Background & aims: Patients with cystic fibrosis (CF) have poorly defined defects in biliary function. We evaluated the effects of cystic fibrosis transmembrane conductance regulator (CFTR) deficiency on the enterohepatic disposition of bile acids (BAs).
Methods: Bile secretion and BA homeostasis were investigated in Cftr(tm1Unc) (Cftr-/-) and CftrΔF508 (ΔF508) mice.
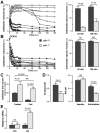
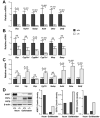
Results: Cftr-/- and ΔF508 mice did not grow to normal size, but did not have liver abnormalities. The gallbladders of Cftr-/- mice were enlarged and had defects in emptying, based on (99m)technetium-mebrofenin scintigraphy or post-prandial variations in gallbladder volume; gallbladder contraction in response to cholecystokinin-8 was normal. Cftr-/- mice had abnormal gallbladder bile and duodenal acidity, and overexpressed the vasoactive intestinal peptide-a myorelaxant factor for the gallbladder. The BA pool was larger in Cftr-/- than wild-type mice, although there were no differences in fecal loss of BAs. Amounts of secondary BAs in portal blood, liver, and bile of Cftr-/- mice were much lower than normal. Expression of genes that are induced by BAs, including fibroblast growth factor-15 and BA transporters, was lower in the ileum but higher in the gallbladders of Cftr-/- mice, compared with wild-type mice, whereas enzymes that synthesize BA were down-regulated in livers of Cftr-/- mice. This indicates that BAs underwent a cholecystohepatic shunt, which was confirmed using cholyl-(Ne-NBD)-lysine as a tracer. In Cftr-/- mice, cholecystectomy reversed most changes in gene expression and partially restored circulating levels of secondary BAs. The ΔF508 mice overexpressed vasoactive intestinal peptide and had defects in gallbladder emptying and in levels of secondary BAs, but these features were less severe than in Cftr-/- mice.
Conclusions: Cftr-/- and CftrΔF508 mice have defects in gallbladder emptying that disrupt enterohepatic circulation of BAs. These defects create a shunt pathway that restricts the amount of toxic secondary BAs that enter the liver.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Comment in
-
Gallbladder: Cftr affects gallbladder function and bile acid homeostasis.Nat Rev Gastroenterol Hepatol. 2012 Mar 20;9(4):188. doi: 10.1038/nrgastro.2012.46. Nat Rev Gastroenterol Hepatol. 2012. PMID: 22429951 No abstract available.
-
A cholecystohepatic shunt pathway: does the gallbladder protect the liver?Gastroenterology. 2012 Jun;142(7):1416-9. doi: 10.1053/j.gastro.2012.04.036. Epub 2012 Apr 26. Gastroenterology. 2012. PMID: 22542828 No abstract available.
-
Bile acid handling in cystic fibrosis: marked phenotypic differences between mouse models.Gastroenterology. 2012 Dec;143(6):e19-20; author reply e20. doi: 10.1053/j.gastro.2012.08.052. Epub 2012 Oct 19. Gastroenterology. 2012. PMID: 23085355 No abstract available.
Similar articles
-
A cholecystohepatic shunt pathway: does the gallbladder protect the liver?Gastroenterology. 2012 Jun;142(7):1416-9. doi: 10.1053/j.gastro.2012.04.036. Epub 2012 Apr 26. Gastroenterology. 2012. PMID: 22542828 No abstract available.
-
The influences of cholecystectomy on the circadian rhythms of bile acids as well as the enterohepatic transporters and enzymes systems in mice.Chronobiol Int. 2018 May;35(5):673-690. doi: 10.1080/07420528.2018.1426596. Epub 2018 Jan 30. Chronobiol Int. 2018. PMID: 29381405
-
Liver X receptor β regulates bile volume and the expression of aquaporins and cystic fibrosis transmembrane conductance regulator in the gallbladder.Am J Physiol Gastrointest Liver Physiol. 2021 Sep 1;321(4):G243-G251. doi: 10.1152/ajpgi.00024.2021. Epub 2021 Jul 14. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 34259574 Free PMC article.
-
Functions of the Gallbladder.Compr Physiol. 2016 Jun 13;6(3):1549-77. doi: 10.1002/cphy.c150050. Compr Physiol. 2016. PMID: 27347902 Review.
-
Bicarbonate secretion in the murine gallbladder--lessons for the treatment of cystic fibrosis.JOP. 2001 Jul;2(4 Suppl):257-62. JOP. 2001. PMID: 11875268 Review.
Cited by
-
Animal models of gastrointestinal and liver diseases. Animal models of cystic fibrosis: gastrointestinal, pancreatic, and hepatobiliary disease and pathophysiology.Am J Physiol Gastrointest Liver Physiol. 2015 Mar 15;308(6):G459-71. doi: 10.1152/ajpgi.00146.2014. Epub 2015 Jan 15. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25591863 Free PMC article. Review.
-
Impaired cholecystokinin-induced gallbladder emptying incriminated in spontaneous "black" pigment gallstone formation in germfree Swiss Webster mice.Am J Physiol Gastrointest Liver Physiol. 2015 Feb 15;308(4):G335-49. doi: 10.1152/ajpgi.00314.2014. Epub 2014 Dec 4. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25477375 Free PMC article.
-
Early intrahepatic duct defects in a cystic fibrosis porcine model.Physiol Rep. 2021 Jul;9(14):e14978. doi: 10.14814/phy2.14978. Physiol Rep. 2021. PMID: 34288572 Free PMC article.
-
Bile Acids and GPBAR-1: Dynamic Interaction Involving Genes, Environment and Gut Microbiome.Nutrients. 2020 Nov 30;12(12):3709. doi: 10.3390/nu12123709. Nutrients. 2020. PMID: 33266235 Free PMC article. Review.
-
Microgallbladder: Self-Remitting Acute Cholecystitis-Like Condition Unique to Patients with Cystic Fibrosis.Case Rep Radiol. 2019 Jun 19;2019:6737428. doi: 10.1155/2019/6737428. eCollection 2019. Case Rep Radiol. 2019. PMID: 31321111 Free PMC article.
References
-
- Hogan DL, Crombie DL, Isenberg JI, et al. Acid-stimulated duodenal bicarbonate secretion involves a CFTR-mediated transport pathway in mice. Gastroenterology. 1997;113:533–541. - PubMed
-
- Chinet T, Fouassier L, Dray-Charier N, et al. Regulation of electrogenic anion secretion in normal and cystic fibrosis gallbladder mucosa. Hepatology. 1999;29:5–13. - PubMed
-
- Zsembery A, Jessner W, Sitter G, et al. Correction of CFTR malfunction and stimulation of Ca-activated Cl channels restore HCO3-secretion in cystic fibrosis bile ductular cells. Hepatology. 2002;35:95–104. - PubMed
-
- Fondacaro JD, Heubi JE, Kellogg FW. Intestinal bile acid malabsorption in cystic fibrosis: a primary mucosal cell defect. Pediatr Res. 1982;16:494–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

