A germinal center-independent pathway generates unswitched memory B cells early in the primary response
- PMID: 22370719
- PMCID: PMC3302224
- DOI: 10.1084/jem.20111696
A germinal center-independent pathway generates unswitched memory B cells early in the primary response
Abstract
Memory B cells can be produced from the classical germinal center (GC) pathway or a less understood GC-independent route. We used antigen-based cell enrichment to assess the relative contributions of these pathways to the polyclonal memory B cell pool. We identified a CD38(+) GL7(+) B cell precursor population that differentiated directly into IgM(+) or isotype-switched (sw) Ig(+) memory B cells in a GC-independent fashion in response to strong CD40 stimulation. Alternatively, CD38(+) GL7(+) B cell precursors had the potential to become Bcl-6(+) GC cells that then generated primarily swIg(+) memory B cells. These results demonstrate that early IgM(+) and swIg(+) memory B cells are products of a GC-independent pathway, whereas later switched Ig(+) memory B cells are products of GC cells.
Figures

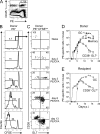
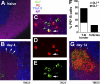




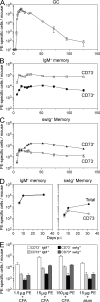

Comment in
-
Literature Watch: implications for transplantation.Am J Transplant. 2013 May;13(5):1117. doi: 10.1111/ajt.12278. Am J Transplant. 2013. PMID: 23621158 No abstract available.
References
-
- Chan T.D., Gatto D., Wood K., Camidge T., Basten A., Brink R. 2009. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J. Immunol. 183:3139–3149 10.4049/jimmunol.0901690 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

