Overexpression of wild-type or mutants forms of CEBPA alter normal human hematopoiesis
- PMID: 22371011
- PMCID: PMC3378638
- DOI: 10.1038/leu.2012.38
Overexpression of wild-type or mutants forms of CEBPA alter normal human hematopoiesis
Abstract
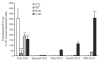
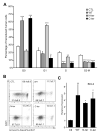
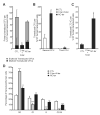
CCAAT/enhancer-binding protein-α (C/EBPα/CEBPA) is mutated in approximately 8% of acute myeloid leukemia (AML) in both familial and sporadic AML and, with FLT3 and NPM1, has received most attention as a predictive marker of outcome in patients with normal karyotype disease. Mutations clustering to either the N- or C-terminal (N- and C-ter) portions of the protein have different consequences on the protein function. In familial cases, the N-ter form is inherited with patients exhibiting long latency period before the onset of overt disease, typically with the acquisition of a C-ter mutation. Despite the essential insights murine models provide the functional consequences of wild-type C/EBPα in human hematopoiesis and how different mutations are involved in AML development have received less attention. Our data underline the critical role of C/EBPα in human hematopoiesis and demonstrate that C/EBPα mutations (alone or in combination) are insufficient to convert normal human hematopoietic stem/progenitor cells into leukemic-initiating cells, although individually each altered normal hematopoiesis. It provides the first insight into the effects of N- and C-ter mutations acting alone and to the combined effects of N/C double mutants. Our results mimicked closely what happens in CEBPA mutated patients.
Figures
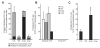

References
-
- Jordan CT, Guzman ML. Mechanisms controlling pathogenesis and survival of leukemic stem cells. Oncogene. 2004;23:7178–7187. - PubMed
-
- Warner JK, Wang JC, Hope KJ, Jin L, Dick JE. Concepts of human leukemic development. Oncogene. 2004;23:7164–7177. - PubMed
-
- Gililand G, Tallman MS. Focus on acute leukemias. Cancer Cell. 2002;1:417–420. - PubMed
-
- Pabst T, Mueller BU. Transcriptional dysregulation during myeloid transformation in AML. Oncogene. 2007;26:6829–6837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

