Tetrahydrobiopterin, L-arginine and vitamin C actsynergistically to decrease oxidative stress, increase nitricoxide and improve blood flow after induction of hindlimbischemia in the rat
- PMID: 22371305
- PMCID: PMC3388126
- DOI: 10.2119/molmed.2011.00103
Tetrahydrobiopterin, L-arginine and vitamin C actsynergistically to decrease oxidative stress, increase nitricoxide and improve blood flow after induction of hindlimbischemia in the rat
Abstract
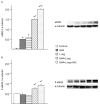
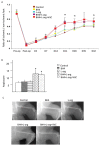
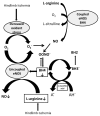
Nitric oxide (NO) derived from endothelial nitric oxide synthase (eNOS) is a potent vasodilator and signaling molecule that plays an essential role in vascular remodeling of collateral arteries and perfusion recovery in response to hindlimb ischemia. In ischemic conditions, decreased NO bioavailability was observed because of increased oxidative stress, decreased L-arginine and tetrahydrobiopterin. This study tested the hypothesis that dietary cosupplementation with tetrahydrobiopterin (BH4), L-arginine, and vitamin C acts synergistically to decrease oxidative stress, increase nitric oxide and improve blood flow in response to acute hindlimb ischemia. Rats were fed normal chow, chow supplemented with BH4 or L-arginine (alone or in combination) or chow supplemented with BH4 + L-arginine + vitamin C for 1 wk before induction of unilateral hindlimb ischemia. Cosupplementation with BH4 + L-arginine resulted in greater eNOS expression, Ca²⁺-dependent NOS activity and NO concentration in gastrocnemius from the ischemic hindlimb, as well as greater recovery of foot perfusion and more collateral artery enlargement than did rats receiving either agent separately. The addition of vitamin C to the BH4 + L-arginine regimen did further increase these dependent variables, although only the increase in eNOS expression reached statistical significances. In addition, rats given all three supplements demonstrated significantly less Ca²⁺-independent activity, less nitrotyrosine accumulation, greater glutathione:glutathione disulfide (GSH:GSSG) ratio and less gastrocnemius muscle necrosis, on both macroscopic and microscopic levels. In conclusion, cosupplementation with BH4 + L-arginine + vitamin C significantly increased vascular perfusion after hindlimb ischemia by increasing eNOS activity and reducing oxidative stress and tissue necrosis. Oral cosupplementation of L-arginine, BH4 and vitamin C holds promise as a biological therapy to induce collateral artery enlargement.
Figures



Similar articles
-
Tetrahydrobiopterin, L-arginine and vitamin C act synergistically to decrease oxidant stress and increase nitric oxide that increases blood flow recovery after hindlimb ischemia in the rat.Mol Med. 2012 Oct 24;18(1):1221-30. doi: 10.2119/molmed.2011.00103.revised. Mol Med. 2012. PMID: 23212846 Free PMC article.
-
Oral tetrahydrobiopterin improves the beneficial effect of adenoviral-mediated eNOS gene transfer after induction of hindlimb ischemia.Mol Ther. 2010 Aug;18(8):1482-9. doi: 10.1038/mt.2010.109. Epub 2010 Jun 15. Mol Ther. 2010. PMID: 20551918 Free PMC article.
-
Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers : evidence for a dysfunctional nitric oxide synthase.Circ Res. 2000 Feb 4;86(2):E36-41. doi: 10.1161/01.res.86.2.e36. Circ Res. 2000. PMID: 10666424
-
L-arginine, tetrahydrobiopterin, nitric oxide and diabetes.Curr Opin Clin Nutr Metab Care. 2013 Jan;16(1):76-82. doi: 10.1097/MCO.0b013e32835ad1ef. Curr Opin Clin Nutr Metab Care. 2013. PMID: 23164986 Review.
-
Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease.Clin Sci (Lond). 2007 Jul;113(2):47-63. doi: 10.1042/CS20070108. Clin Sci (Lond). 2007. PMID: 17555404 Review.
Cited by
-
Coronary artery spasm related to thiol oxidation and senescence marker protein-30 in aging.Antioxid Redox Signal. 2013 Oct 1;19(10):1063-73. doi: 10.1089/ars.2012.4903. Epub 2013 Feb 19. Antioxid Redox Signal. 2013. PMID: 23320823 Free PMC article.
-
Nutraceuticals as Modulators of Molecular Placental Pathways: Their Potential to Prevent and Support the Treatment of Preeclampsia.Int J Mol Sci. 2024 Nov 13;25(22):12167. doi: 10.3390/ijms252212167. Int J Mol Sci. 2024. PMID: 39596234 Free PMC article. Review.
-
Vitamin C and Cardiovascular Disease: An Update.Antioxidants (Basel). 2020 Dec 3;9(12):1227. doi: 10.3390/antiox9121227. Antioxidants (Basel). 2020. PMID: 33287462 Free PMC article. Review.
-
Combined oral administration of L-arginine and tetrahydrobiopterin in a rat model of pulmonary arterial hypertension.Pulm Circ. 2017 Apr 4;7(1):89-97. doi: 10.1086/689289. eCollection 2017 Mar. Pulm Circ. 2017. PMID: 28680568 Free PMC article.
-
Vitamin C: A Comprehensive Review of Its Role in Health, Disease Prevention, and Therapeutic Potential.Molecules. 2025 Feb 6;30(3):748. doi: 10.3390/molecules30030748. Molecules. 2025. PMID: 39942850 Free PMC article. Review.
References
-
- Silvestro A, Oliva G, Brevetti G. Intermittent claudication and endothelial dysfunction. Eur Heart J. 2002;4:B35–40.
-
- Marletta M. Nitric oxide synthase structure and mechanism. J Biol. Chem. 1993;268:12231–12234. - PubMed
-
- Peterson T, et al. Opposing effects of reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. Circ Res. 1999;85:29–37. - PubMed
-
- Kuzkaya N, Weissman N, Harrison D, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols. J Biol Chem. 2003;278:22546–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

