Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-α-induced shock
- PMID: 22371307
- PMCID: PMC3388137
- DOI: 10.2119/molmed.2011.00423
Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-α-induced shock
Abstract
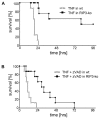
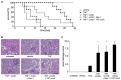
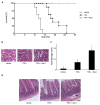
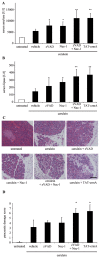
Tumor necrosis factor receptor (TNFR) signaling may result in survival, apoptosis or programmed necrosis. The latter is called necroptosis if the receptor-interacting protein 1 (RIP1) inhibitor necrostatin-1 (Nec-1) or genetic knockout of RIP3 prevents it. In the lethal mouse model of TNFα-mediated shock, addition of the pan-caspase inhibitor zVAD-fmk (zVAD) accelerates time to death. Here, we demonstrate that RIP3-deficient mice are protected markedly from TNFα-mediated shock in the presence and absence of caspase inhibition. We further show that the fusion protein TAT-crmA, previously demonstrated to inhibit apoptosis, also prevents necroptosis in L929, HT29 and FADD-deficient Jurkat cells. In contrast to RIP3-deficient mice, blocking necroptosis by Nec-1 or TAT-crmA did not protect from TNFα/zVAD-mediated shock, but further accelerated time to death. Even in the absence of caspase inhibition, Nec-1 application led to similar kinetics. Depletion of macrophages, natural killer (NK) cells, granulocytes or genetic deficiency for T lymphocytes did not influence this model. Because RIP3-deficient mice are known to be protected from cerulein-induced pancreatitis (CIP), we applied Nec-1 and TAT-crmA in this model and demonstrated the deterioration of pancreatic damage upon addition of these substances. These data highlight the importance of separating genetic RIP3 deficiency from RIP1 inhibition by Nec-1 application in vivo and challenge the current definition of necroptosis.
Figures

Similar articles
-
RIP1-dependent and independent effects of necrostatin-1 in necrosis and T cell activation.PLoS One. 2011;6(8):e23209. doi: 10.1371/journal.pone.0023209. Epub 2011 Aug 10. PLoS One. 2011. PMID: 21853090 Free PMC article.
-
Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia.Cell Death Dis. 2013 Jul 11;4(7):e716. doi: 10.1038/cddis.2013.238. Cell Death Dis. 2013. PMID: 23846218 Free PMC article.
-
Distinctive roles of receptor-interacting protein kinases 1 and 3 in caspase-independent cell death of L929.Cell Biochem Funct. 2014 Jan;32(1):62-9. doi: 10.1002/cbf.2972. Epub 2013 Apr 15. Cell Biochem Funct. 2014. PMID: 23584955
-
Necrosis-dependent and independent signaling of the RIP kinases in inflammation.Cytokine Growth Factor Rev. 2014 Apr;25(2):167-74. doi: 10.1016/j.cytogfr.2013.12.013. Epub 2013 Dec 25. Cytokine Growth Factor Rev. 2014. PMID: 24412261 Free PMC article. Review.
-
Necroptosis in health and diseases.Semin Cell Dev Biol. 2014 Nov;35:14-23. doi: 10.1016/j.semcdb.2014.07.013. Epub 2014 Aug 1. Semin Cell Dev Biol. 2014. PMID: 25087983 Review.
Cited by
-
TLR3, TRIF, and caspase 8 determine double-stranded RNA-induced epithelial cell death and survival in vivo.J Immunol. 2013 Jan 1;190(1):418-27. doi: 10.4049/jimmunol.1202756. Epub 2012 Dec 3. J Immunol. 2013. PMID: 23209324 Free PMC article.
-
Fight or flight: regulation of emergency hematopoiesis by pyroptosis and necroptosis.Curr Opin Hematol. 2015 Jul;22(4):293-301. doi: 10.1097/MOH.0000000000000148. Curr Opin Hematol. 2015. PMID: 26049749 Free PMC article. Review.
-
RIPK3-Mediated Necroptosis and Neutrophil Infiltration Are Associated with Poor Prognosis in Patients with Alcoholic Cirrhosis.J Immunol Res. 2018 Nov 25;2018:1509851. doi: 10.1155/2018/1509851. eCollection 2018. J Immunol Res. 2018. PMID: 30596105 Free PMC article.
-
Regulated necrosis: disease relevance and therapeutic opportunities.Nat Rev Drug Discov. 2016 May;15(5):348-66. doi: 10.1038/nrd.2015.6. Epub 2016 Jan 18. Nat Rev Drug Discov. 2016. PMID: 26775689 Free PMC article. Review.
-
Necroptosis in development, inflammation and disease.Nat Rev Mol Cell Biol. 2017 Feb;18(2):127-136. doi: 10.1038/nrm.2016.149. Epub 2016 Dec 21. Nat Rev Mol Cell Biol. 2017. PMID: 27999438 Review.
References
-
- Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–95. - PubMed
-
- Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

