Sarcoplasmic reticulum Ca2+ permeation explored from the lumen side in mdx muscle fibers under voltage control
- PMID: 22371362
- PMCID: PMC3289961
- DOI: 10.1085/jgp.201110738
Sarcoplasmic reticulum Ca2+ permeation explored from the lumen side in mdx muscle fibers under voltage control
Abstract
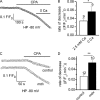
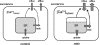
Under resting conditions, external Ca(2+) is known to enter skeletal muscle cells, whereas Ca(2+) stored in the sarcoplasmic reticulum (SR) leaks into the cytosol. The nature of the pathways involved in the sarcolemmal Ca(2+) entry and in the SR Ca(2+) leak is still a matter of debate, but several lines of evidence suggest that these Ca(2+) fluxes are up-regulated in Duchenne muscular dystrophy. We investigated here SR calcium permeation at resting potential and in response to depolarization in voltage-controlled skeletal muscle fibers from control and mdx mice, the mouse model of Duchenne muscular dystrophy. Using the cytosolic Ca(2+) dye Fura2, we first demonstrated that the rate of Ca(2+) increase in response to cyclopiazonic acid (CPA)-induced inhibition of SR Ca(2+)-ATPases at resting potential was significantly higher in mdx fibers, which suggests an elevated SR Ca(2+) leak. However, removal of external Ca(2+) reduced the rate of CPA-induced Ca(2+) increase in mdx and increased it in control fibers, which indicates an up-regulation of sarcolemmal Ca(2+) influx in mdx fibers. Fibers were then loaded with the low-affinity Ca(2+) dye Fluo5N-AM to measure intraluminal SR Ca(2+) changes. Trains of action potentials, chloro-m-cresol, and depolarization pulses evoked transient Fluo5N fluorescence decreases, and recovery of voltage-induced Fluo5N fluorescence changes were inhibited by CPA, demonstrating that Fluo5N actually reports intraluminal SR Ca(2+) changes. Voltage dependence and magnitude of depolarization-induced SR Ca(2+) depletion were found to be unchanged in mdx fibers, but the rate of the recovery phase that followed depletion was found to be faster, indicating a higher SR Ca(2+) reuptake activity in mdx fibers. Overall, CPA-induced SR Ca(2+) leak at -80 mV was found to be significantly higher in mdx fibers and was potentiated by removal of external Ca(2+) in control fibers. The elevated passive SR Ca(2+) leak may contribute to alteration of Ca(2+) homeostasis in mdx muscle.
© 2012 Robin et al.
Figures





Similar articles
-
Sarcoplasmic reticulum Ca2+ release and depletion fail to affect sarcolemmal ion channel activity in mouse skeletal muscle.J Physiol. 2006 Aug 15;575(Pt 1):69-81. doi: 10.1113/jphysiol.2006.112367. Epub 2006 Jun 15. J Physiol. 2006. PMID: 16777939 Free PMC article.
-
Effect of cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca-ATPase, on skeletal muscles from normal and mdx mice.Acta Physiol Scand. 2005 Jul;184(3):173-86. doi: 10.1111/j.1365-201X.2005.01450.x. Acta Physiol Scand. 2005. PMID: 15954985
-
Ca(2+) influx and opening of Ca(2+)-activated K(+) channels in muscle fibers from control and mdx mice.Biophys J. 2002 Jun;82(6):3012-21. doi: 10.1016/S0006-3495(02)75642-7. Biophys J. 2002. PMID: 12023224 Free PMC article.
-
A study of the mechanisms of excitation-contraction coupling in frog skeletal muscle based on measurements of [Ca2+] transients inside the sarcoplasmic reticulum.J Muscle Res Cell Motil. 2018 Apr;39(1-2):41-60. doi: 10.1007/s10974-018-9497-9. Epub 2018 Aug 24. J Muscle Res Cell Motil. 2018. PMID: 30143958 Review.
-
Molecular architecture of Ca2+ signaling control in muscle and heart cells.Channels (Austin). 2011 Sep-Oct;5(5):391-6. doi: 10.4161/chan.5.5.16467. Epub 2011 Sep 1. Channels (Austin). 2011. PMID: 21712647 Review.
Cited by
-
Dynamic measurement of the calcium buffering properties of the sarcoplasmic reticulum in mouse skeletal muscle.J Physiol. 2013 Jan 15;591(2):423-42. doi: 10.1113/jphysiol.2012.243444. Epub 2012 Nov 12. J Physiol. 2013. PMID: 23148320 Free PMC article.
-
Abnormal Calcium Handling in Duchenne Muscular Dystrophy: Mechanisms and Potential Therapies.Front Physiol. 2021 Apr 9;12:647010. doi: 10.3389/fphys.2021.647010. eCollection 2021. Front Physiol. 2021. PMID: 33897454 Free PMC article. Review.
-
Voltage-gated Ca(2+) influx through L-type channels contributes to sarcoplasmic reticulum Ca(2+) loading in skeletal muscle.J Physiol. 2015 Nov 1;593(21):4781-97. doi: 10.1113/JP270252. Epub 2015 Oct 18. J Physiol. 2015. PMID: 26383921 Free PMC article.
-
Increased sarcolipin expression and decreased sarco(endo)plasmic reticulum Ca2+ uptake in skeletal muscles of mouse models of Duchenne muscular dystrophy.J Muscle Res Cell Motil. 2013 Dec;34(5-6):349-56. doi: 10.1007/s10974-013-9350-0. Epub 2013 Jun 8. J Muscle Res Cell Motil. 2013. PMID: 23748997
-
Spaceflight increases sarcoplasmic reticulum Ca2+ leak and this cannot be counteracted with BuOE treatment.NPJ Microgravity. 2024 Jul 19;10(1):78. doi: 10.1038/s41526-024-00419-y. NPJ Microgravity. 2024. PMID: 39030182 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

