Molecular and biophysical basis of glutamate and trace metal modulation of voltage-gated Ca(v)2.3 calcium channels
- PMID: 22371363
- PMCID: PMC3289959
- DOI: 10.1085/jgp.201110699
Molecular and biophysical basis of glutamate and trace metal modulation of voltage-gated Ca(v)2.3 calcium channels
Erratum in
- J Gen Physiol. 2012 May;139(5):389
Abstract
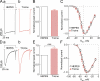
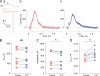
Here, we describe a new mechanism by which glutamate (Glu) and trace metals reciprocally modulate activity of the Ca(v)2.3 channel by profoundly shifting its voltage-dependent gating. We show that zinc and copper, at physiologically relevant concentrations, occupy an extracellular binding site on the surface of Ca(v)2.3 and hold the threshold for activation of these channels in a depolarized voltage range. Abolishing this binding by chelation or the substitution of key amino acid residues in IS1-IS2 (H111) and IS2-IS3 (H179 and H183) loops potentiates Ca(v)2.3 by shifting the voltage dependence of activation toward more negative membrane potentials. We demonstrate that copper regulates the voltage dependence of Ca(v)2.3 by affecting gating charge movements. Thus, in the presence of copper, gating charges transition into the "ON" position slower, delaying activation and reducing the voltage sensitivity of the channel. Overall, our results suggest a new mechanism by which Glu and trace metals transiently modulate voltage-dependent gating of Ca(v)2.3, potentially affecting synaptic transmission and plasticity in the brain.
© 2012 Shcheglovitov et al.
Figures


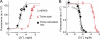






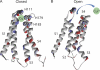
Similar articles
-
Reciprocal modulation of Cav 2.3 voltage-gated calcium channels by copper(II) ions and kainic acid.J Neurochem. 2018 Nov;147(3):310-322. doi: 10.1111/jnc.14546. Epub 2018 Aug 30. J Neurochem. 2018. PMID: 29972687
-
Swapping the I-II intracellular linker between L-type CaV1.2 and R-type CaV2.3 high-voltage gated calcium channels exchanges activation attributes.Channels (Austin). 2010 Jan-Feb;4(1):42-50. doi: 10.4161/chan.4.1.10562. Epub 2010 Jan 9. Channels (Austin). 2010. PMID: 20026913
-
Two sets of amino acids of the domain I of Cav2.3 Ca(2+) channels contribute to their high sensitivity to extracellular protons.Pflugers Arch. 2011 Aug;462(2):303-14. doi: 10.1007/s00424-011-0974-x. Epub 2011 May 25. Pflugers Arch. 2011. PMID: 21611731
-
Voltage-dependent calcium channels.Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review.
-
Trace metals in the brain: allosteric modulators of ligand-gated receptor channels, the case of ATP-gated P2X receptors.Eur Biophys J. 2008 Mar;37(3):301-14. doi: 10.1007/s00249-007-0230-7. Epub 2007 Oct 31. Eur Biophys J. 2008. PMID: 17972073 Review.
Cited by
-
Further Evidence that Inhibition of Neuronal Voltage-Gated Calcium Channels Contributes to the Hypnotic Effect of Neurosteroid Analogue, 3β-OH.Front Pharmacol. 2022 May 23;13:850658. doi: 10.3389/fphar.2022.850658. eCollection 2022. Front Pharmacol. 2022. PMID: 35677453 Free PMC article.
-
Cav2.3 channel function and Zn2+-induced modulation: potential mechanisms and (patho)physiological relevance.Channels (Austin). 2020 Dec;14(1):362-379. doi: 10.1080/19336950.2020.1829842. Channels (Austin). 2020. PMID: 33079629 Free PMC article. Review.
-
Complex modulation of Ca(v)3.1 T-type calcium channel by nickel.Cell Mol Life Sci. 2013 May;70(9):1653-61. doi: 10.1007/s00018-012-1225-9. Epub 2012 Dec 19. Cell Mol Life Sci. 2013. PMID: 23250353 Free PMC article.
-
Intracerebroventricular administration of histidine reduces kainic acid-induced convulsive seizures in mice.Exp Brain Res. 2019 Oct;237(10):2481-2493. doi: 10.1007/s00221-019-05605-z. Epub 2019 Jul 18. Exp Brain Res. 2019. PMID: 31321447
-
Copper signalling: causes and consequences.Cell Commun Signal. 2018 Oct 22;16(1):71. doi: 10.1186/s12964-018-0277-3. Cell Commun Signal. 2018. PMID: 30348177 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

