Defective retinal depolarizing bipolar cells in regulators of G protein signaling (RGS) 7 and 11 double null mice
- PMID: 22371490
- PMCID: PMC3340290
- DOI: 10.1074/jbc.M112.345751
Defective retinal depolarizing bipolar cells in regulators of G protein signaling (RGS) 7 and 11 double null mice
Abstract
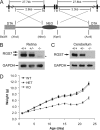
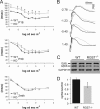
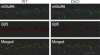
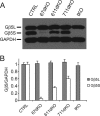
Two members of the R7 subfamily of regulators of G protein signaling, RGS7 and RGS11, are present at dendritic tips of retinal depolarizing bipolar cells (DBCs). Their involvement in the mGluR6/Gα(o)/TRPM1 pathway that mediates DBC light responses has been implicated. However, previous genetic studies employed an RGS7 mutant mouse that is hypomorphic, and hence the exact role of RGS7 in DBCs remains unclear. We have made a true RGS7-null mouse line with exons 6-8 deleted. The RGS7(-/-) mouse is viable and fertile but smaller in body size. Electroretinogram (ERG) b-wave implicit time in young RGS7(-/-) mice is prolonged at eye opening, but the phenotype disappears at 2 months of age. Expression levels of RGS6 and RGS11 are unchanged in RGS7(-/-) retina, but the Gβ5S level is significantly reduced. By characterizing a complete RGS7 and RGS11 double knock-out (711dKO) mouse line, we found that Gβ5S expression in the retinal outer plexiform layer is eliminated, as is the ERG b-wave. Ultrastructural defects akin to those of Gβ5(-/-) mice are evident in 711dKO mice. In retinas of mice lacking RGS6, RGS7, and RGS11, Gβ5S is undetectable, whereas levels of the photoreceptor-specific Gβ5L remain unchanged. Whereas RGS6 alone sustains a significant amount of Gβ5S expression in retina, the DBC-related defects in Gβ5(-/-) mice are caused solely by a combined loss of RGS7 and RGS11. Our data support the notion that the role of Gβ5 in the retina, and likely in the entire nervous system, is mediated exclusively by R7 RGS proteins.
Figures

References
-
- Ross E. M., Wilkie T. M. (2000) GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69, 795–827 - PubMed
-
- Snow B. E., Krumins A. M., Brothers G. M., Lee S. F., Wall M. A., Chung S., Mangion J., Arya S., Gilman A. G., Siderovski D. P. (1998) A G protein γ subunit-like domain shared between RGS11 and other RGS proteins specifies binding to Gβ5 subunits. Proc. Natl. Acad. Sci. U.S.A. 95, 13307–13312 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

