Myocilin interacts with syntrophins and is member of dystrophin-associated protein complex
- PMID: 22371502
- PMCID: PMC3339941
- DOI: 10.1074/jbc.M111.224063
Myocilin interacts with syntrophins and is member of dystrophin-associated protein complex
Abstract
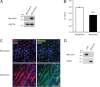
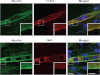
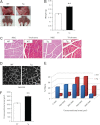
Genetic studies have linked myocilin to open angle glaucoma, but the functions of the protein in the eye and other tissues have remained elusive. The purpose of this investigation was to elucidate myocilin function(s). We identified α1-syntrophin, a component of the dystrophin-associated protein complex (DAPC), as a myocilin-binding candidate. Myocilin interacted with α1-syntrophin via its N-terminal domain and co-immunoprecipitated with α1-syntrophin from C2C12 myotubes and mouse skeletal muscle. Expression of 15-fold higher levels of myocilin in the muscles of transgenic mice led to the elevated association of α1-syntrophin, neuronal nitric-oxide synthase, and α-dystroglycan with DAPC, which increased the binding of laminin to α-dystroglycan and Akt signaling. Phosphorylation of Akt and Forkhead box O-class 3, key regulators of muscle size, was increased more than 3-fold, whereas the expression of muscle-specific RING finger protein-1 and atrogin-1, muscle atrophy markers, was decreased by 79 and 88%, respectively, in the muscles of transgenic mice. Consequently, the average size of muscle fibers of the transgenic mice was increased by 36% relative to controls. We suggest that intracellular myocilin plays a role as a regulator of muscle hypertrophy pathways, acting through the components of DAPC.
Figures




Similar articles
-
Differential association of syntrophin pairs with the dystrophin complex.J Cell Biol. 1997 Jul 14;138(1):81-93. doi: 10.1083/jcb.138.1.81. J Cell Biol. 1997. PMID: 9214383 Free PMC article.
-
Regulation of TRPC1 and TRPC4 cation channels requires an alpha1-syntrophin-dependent complex in skeletal mouse myotubes.J Biol Chem. 2009 Dec 25;284(52):36248-36261. doi: 10.1074/jbc.M109.012872. Epub 2009 Oct 7. J Biol Chem. 2009. PMID: 19812031 Free PMC article.
-
Dystroglycan is not required for localization of dystrophin, syntrophin, and neuronal nitric-oxide synthase at the sarcolemma but regulates integrin alpha 7B expression and caveolin-3 distribution.J Biol Chem. 2002 Feb 15;277(7):4672-9. doi: 10.1074/jbc.M106879200. Epub 2001 Dec 6. J Biol Chem. 2002. PMID: 11741881
-
Dystrophin complex functions as a scaffold for signalling proteins.Biochim Biophys Acta. 2014 Feb;1838(2):635-42. doi: 10.1016/j.bbamem.2013.08.023. Epub 2013 Sep 7. Biochim Biophys Acta. 2014. PMID: 24021238 Review.
-
Dystrophin and utrophin: genetic analyses of their role in skeletal muscle.Microsc Res Tech. 2000 Feb 1-15;48(3-4):155-66. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<155::AID-JEMT4>3.0.CO;2-0. Microsc Res Tech. 2000. PMID: 10679963 Review.
Cited by
-
Myocilin mediates myelination in the peripheral nervous system through ErbB2/3 signaling.J Biol Chem. 2013 Sep 13;288(37):26357-71. doi: 10.1074/jbc.M112.446138. Epub 2013 Jul 29. J Biol Chem. 2013. PMID: 23897819 Free PMC article.
-
Novel γ-sarcoglycan interactors in murine muscle membranes.Skelet Muscle. 2022 Jan 22;12(1):2. doi: 10.1186/s13395-021-00285-2. Skelet Muscle. 2022. PMID: 35065666 Free PMC article.
-
Trifunctional High-Throughput Screen Identifies Promising Scaffold To Inhibit Grp94 and Treat Myocilin-Associated Glaucoma.ACS Chem Biol. 2018 Apr 20;13(4):933-941. doi: 10.1021/acschembio.7b01083. Epub 2018 Feb 20. ACS Chem Biol. 2018. PMID: 29402077 Free PMC article.
-
Transgenic Overexpression of Myocilin Leads to Variable Ocular Anterior Segment and Retinal Alterations Associated with Extracellular Matrix Abnormalities in Adult Zebrafish.Int J Mol Sci. 2022 Sep 1;23(17):9989. doi: 10.3390/ijms23179989. Int J Mol Sci. 2022. PMID: 36077382 Free PMC article.
-
A role for myocilin in receptor-mediated endocytosis.PLoS One. 2013 Dec 18;8(12):e82301. doi: 10.1371/journal.pone.0082301. eCollection 2013. PLoS One. 2013. PMID: 24367514 Free PMC article.
References
-
- Polansky J. R., Fauss D. J., Chen P., Chen H., Lütjen-Drecoll E., Johnson D., Kurtz R. M., Ma Z. D., Bloom E., Nguyen T. D. (1997) Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica 211, 126–139 - PubMed
-
- Nguyen T. D., Chen P., Huang W. D., Chen H., Johnson D., Polansky J. R. (1998) Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J. Biol. Chem. 273, 6341–6350 - PubMed
-
- Fautsch M. P., Johnson D. H. (2001) Characterization of myocilin-myocilin interactions. Invest. Ophthalmol. Vis. Sci. 42, 2324–2331 - PubMed
-
- Torrado M., Trivedi R., Zinovieva R., Karavanova I., Tomarev S. I. (2002) Optimedin. A novel olfactomedin-related protein that interacts with myocilin. Hum. Mol. Genet. 11, 1291–1301 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

