Subclassification and biochemical analysis of plant papain-like cysteine proteases displays subfamily-specific characteristics
- PMID: 22371507
- PMCID: PMC3320171
- DOI: 10.1104/pp.112.194001
Subclassification and biochemical analysis of plant papain-like cysteine proteases displays subfamily-specific characteristics
Abstract
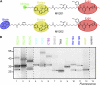
Papain-like cysteine proteases (PLCPs) are a large class of proteolytic enzymes associated with development, immunity, and senescence. Although many properties have been described for individual proteases, the distribution of these characteristics has not been studied collectively. Here, we analyzed 723 plant PLCPs and classify them into nine subfamilies that are present throughout the plant kingdom. Analysis of these subfamilies revealed previously unreported distinct subfamily-specific functional and structural characteristics. For example, the NPIR and KDEL localization signals are distinctive for subfamilies, and the carboxyl-terminal granulin domain occurs in two PLCP subfamilies, in which some individual members probably evolved by deletion of the granulin domains. We also discovered a conserved double cysteine in the catalytic site of SAG12-like proteases and two subfamily-specific disulfides in RD19A-like proteases. Protease activity profiling of representatives of the PLCP subfamilies using novel fluorescent probes revealed striking polymorphic labeling profiles and remarkably distinct pH dependency. Competition assays with peptide-epoxide scanning libraries revealed common and unique inhibitory fingerprints. Finally, we expand the detection of PLCPs by identifying common and organ-specific protease activities and identify previously undetected proteases upon labeling with cell-penetrating probes in vivo. This study provides the plant protease research community with tools for further functional annotation of plant PLCPs.
Figures






References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 - PubMed
-
- Avci U, Petzold HE, Ismail IO, Beers EP, Haigler CH. (2008) Cysteine proteases XCP1 and XCP2 aid micro-autolysis within the intact central vacuole during xylogenesis in Arabidopsis roots. Plant J 56: 303–315 - PubMed
-
- Baldauf SL. (2003) Phylogeny for the faint of heart: a tutorial. Trends Genet 19: 345–351 - PubMed
-
- Bateman A, Bennett HPJ. (2009) The granulin gene family: from cancer to dementia. Bioessays 31: 1245–1254 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

