Water reservoir maintained by cell growth fuels the spreading of a bacterial swarm
- PMID: 22371567
- PMCID: PMC3306679
- DOI: 10.1073/pnas.1118238109
Water reservoir maintained by cell growth fuels the spreading of a bacterial swarm
Abstract
Flagellated bacteria can swim across moist surfaces within a thin layer of fluid, a means for surface colonization known as swarming. This fluid spreads with the swarm, but how it does so is unclear. We used micron-sized air bubbles to study the motion of this fluid within swarms of Escherichia coli. The bubbles moved diffusively, with drift. Bubbles starting at the swarm edge drifted inward for the first 5 s and then moved outward. Bubbles starting 30 μm from the swarm edge moved inward for the first 20 s, wandered around in place for the next 40 s, and then moved outward. Bubbles starting at 200 or 300 μm from the edge moved outward or wandered around in place, respectively. So the general trend was inward near the outer edge of the swarm and outward farther inside, with flows converging on a region about 100 μm from the swarm edge. We measured cellular metabolic activities with cells expressing a short-lived GFP and cell densities with cells labeled with a membrane fluorescent dye. The fluorescence plots were similar, with peaks about 80 μm from the swarm edge and slopes that mimicked the particle drift rates. These plots suggest that net fluid flow is driven by cell growth. Fluid depth is largest in the multilayered region between approximately 30 and 200 μm from the swarm edge, where fluid agitation is more vigorous. This water reservoir travels with the swarm, fueling its spreading. Intercellular communication is not required; cells need only grow.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
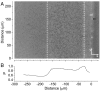
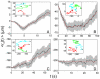
 , black solid line] of bubble trajectories at different regions inside the swarm, shown as a function of time. Linear fits to
, black solid line] of bubble trajectories at different regions inside the swarm, shown as a function of time. Linear fits to  , indicating radial drift velocities, vx, are shown by red dashed lines. The gray areas indicate standard errors in the mean. The insets show typical bubble tracks in the laboratory frame measured in μm, beginning at + and ending at x. Tracking began at distances from the swarm edge shown on the ordinates at t = 0 and continued over the time span shown on the abscissas. The numbers of tracks analyzed were (A) 29, (B) 35, (C) 43, and (D) 22. Data analysis was continued until about half of the trajectories extended beyond the region of interest; e.g., the cell monolayer, A; the multilayered region, B; and the regions beyond the multilayered region, C and D.
, indicating radial drift velocities, vx, are shown by red dashed lines. The gray areas indicate standard errors in the mean. The insets show typical bubble tracks in the laboratory frame measured in μm, beginning at + and ending at x. Tracking began at distances from the swarm edge shown on the ordinates at t = 0 and continued over the time span shown on the abscissas. The numbers of tracks analyzed were (A) 29, (B) 35, (C) 43, and (D) 22. Data analysis was continued until about half of the trajectories extended beyond the region of interest; e.g., the cell monolayer, A; the multilayered region, B; and the regions beyond the multilayered region, C and D.
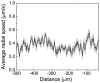
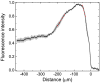
References
-
- Harshey RM. Bacterial motility on a surface: Many ways to a common goal. Annu Rev Microbiol. 2003;57:249–273. - PubMed
-
- Koch DL, Subramanian G. Collective hydrodynamics of swimming microorganisms: Living fluids. Annu Rev Fluid Mech. 2011;43:637–659.
-
- Ramaswamy S. The mechanics and statistics of active matter. Annu Rev Condens Matter Phys. 2010;1:323–345.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

