Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure
- PMID: 22371878
- PMCID: PMC4916559
- DOI: 10.1182/blood-2011-11-390120
Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure
Abstract
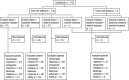
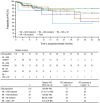
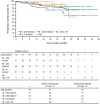
Bosutinib, a dual Src/Abl tyrosine kinase inhibitor (TKI), has shown potent activity against chronic myeloid leukemia (CML). This phase 1/2 study evaluated the efficacy and safety of once-daily bosutinib 500 mg in leukemia patients after resistance/intolerance to imatinib. The current analysis included 118 patients with chronic-phase CML who had been pretreated with imatinib followed by dasatinib and/or nilotinib, with a median follow-up of 28.5 months. In this subpopulation, major cytogenetic response was attained by 32% of patients; complete cytogenetic response was attained by 24%, including in one of 3 patients treated with 3 prior TKIs. Complete hematologic response was achieved/maintained in 73% of patients. On-treatment transformation to accelerated/blast phase occurred in 5 patients. At 2 years, Kaplan-Meier-estimated progression-free survival was 73% and estimated overall survival was 83%. Responses were seen across Bcr-Abl mutations, including those associated with dasatinib and nilotinib resistance, except T315I. Bosutinib had an acceptable safety profile; treatment-emergent adverse events were primarily manageable grade 1/2 gastrointestinal events and rash. Grade 3/4 nonhematologic adverse events (> 2% of patients) included diarrhea (8%) and rash (4%). Bosutinib may offer a new treatment option for patients with chronic-phase CML after treatment with multiple TKIs. This trial was registered at www.clinicaltrials.gov as NCT00261846.
Figures

References
-
- Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341(3):164–172. - PubMed
-
- de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26(20):3358–3363. - PubMed
-
- O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. - PubMed
-
- Ramirez P, Dipersio JF. Therapy options in imatinib failures. Oncologist. 2008;13(4):424–434. - PubMed
-
- Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer. 2007;7(5):345–356. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

