Phase 2 study of rituximab plus ABVD in patients with newly diagnosed classical Hodgkin lymphoma
- PMID: 22371887
- PMCID: PMC3359733
- DOI: 10.1182/blood-2012-01-405456
Phase 2 study of rituximab plus ABVD in patients with newly diagnosed classical Hodgkin lymphoma
Abstract
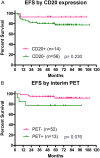
In the present study, we evaluated the efficacy and safety of rituximab in combination with standard doxorubicin, bleomycin, vinblastine, and dacarbazine (RABVD) in patients with classical Hodgkin lymphoma (cHL). In this phase 2 study, patients with chemotherapy-naive, advanced-stage cHL were treated with rituximab 375 mg/m(2) weekly for 6 weeks and standard ABVD for 6 cycles. The primary outcome was event-free survival (EFS) at 5 years. Eighty-five patients were enrolled, of whom 78 were eligible. With a median follow-up duration of 68 months (range, 26-110), and based on an intent-to-treat analysis, the 5-year EFS and overall survival rates were 83% and 96%, respectively. The 5-year EFS for patients with stage III/IV cHL was 82%. Furthermore, the 5-year EFS for patients with an International Prognostic Score of 0-2 was 88% and for those with a score of > 2, it was 73%. The most frequent treatment-related grade 3 or 4 adverse events were neutropenia (23%), fatigue (9%), and nausea (8%). Our results demonstrate that the addition of rituximab to ABVD is safe and has a promising clinical activity in patients with advanced-stage cHL. These data are currently being confirmed in a multicenter randomized trial.
Trial registration: ClinicalTrials.gov NCT00504504.
Figures


Comment in
-
Off-targeting oft-targeted CD20 in cHL.Blood. 2012 May 3;119(18):4095-6. doi: 10.1182/blood-2012-03-413146. Blood. 2012. PMID: 22555658
References
-
- Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327(21):1478–1484. - PubMed
-
- Connors JM. State-of-the-art therapeutics: Hodgkin's lymphoma. J Clin Oncol. 2005;23(26):6400–6408. - PubMed
-
- Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin's lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27(27):4548–4554. - PubMed
-
- Viviani S, Zinzani PL, Rambaldi A, et al. ABVD versus BEACOPP for Hodgkin's lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365(3):203–212. - PubMed
-
- Evens AM, Hutchings M, Diehl V. Treatment of Hodgkin lymphoma: the past, present, and future. Nat Clin Pract Oncol. 2008;5(9):543–556. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

