Population pharmacokinetic analysis and pharmacogenetics of raltegravir in HIV-positive and healthy individuals
- PMID: 22371894
- PMCID: PMC3370715
- DOI: 10.1128/AAC.05424-11
Population pharmacokinetic analysis and pharmacogenetics of raltegravir in HIV-positive and healthy individuals
Abstract
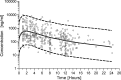
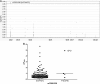
The objectives of this study were to characterize raltegravir (RAL) population pharmacokinetics in HIV-positive (HIV(+)) and healthy individuals, identify influential factors, and search for new candidate genes involved in UDP glucuronosyltransferase (UGT)-mediated glucuronidation. The pharmacokinetic analysis was performed with NONMEM. Genetic association analysis was performed with PLINK using the relative bioavailability as the phenotype. Simulations were performed to compare once- and twice-daily regimens. A 2-compartment model with first-order absorption adequately described the data. Atazanavir, gender, and bilirubin levels influenced RAL relative bioavailability, which was 30% lower in HIV(+) than in healthy individuals. UGT1A9*3 was the only genetic variant possibly influencing RAL pharmacokinetics. The majority of RAL pharmacokinetic variability remains unexplained by genetic and nongenetic factors. Owing to the very large variability, trough drug levels might be very low under the standard dosing regimen, raising the question of a potential relevance of therapeutic drug monitoring of RAL in some situations.
Figures

References
-
- Ashby J, et al. 2011. Pharmacokinetic and safety profile of raltegravir and ribavirin, when dosed separately and together, in healthy volunteers. J. Antimicrob. Chemother. 66:1340–1345 - PubMed
-
- Baroncelli S, et al. 2010. Raltegravir plasma concentrations in treatment-experienced patients receiving salvage regimens based on raltegravir with and without maraviroc coadministration. Ann. Pharmacother. 44:838–843 - PubMed
-
- Bernard O, Guillemette C. 2004. The main role of UGT1A9 in the hepatic metabolism of mycophenolic acid and the effects of naturally occurring variants. Drug Metab. Dispos. 32:775–778 - PubMed
-
- Brainard DM, et al. 2011. Effect of low-, moderate-, and high-fat meals on raltegravir pharmacokinetics. J. Clin. Pharmacol. 51:422–427 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

