Dual peptide nucleic acid- and peptide-functionalized shell cross-linked nanoparticles designed to target mRNA toward the diagnosis and treatment of acute lung injury
- PMID: 22372643
- PMCID: PMC3321742
- DOI: 10.1021/bc200629f
Dual peptide nucleic acid- and peptide-functionalized shell cross-linked nanoparticles designed to target mRNA toward the diagnosis and treatment of acute lung injury
Abstract
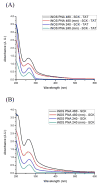
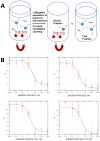
In this work, multifunctional biosynthetic hybrid nanostructures were prepared and studied for their potential utility in the recognition and inhibition of mRNA sequences for inducible nitric oxide synthase (iNOS), which are overexpressed at sites of inflammation, such as in cases of acute lung injury. Shell cross-linked knedel-like polymer nanoparticles (SCKs) that present peptide nucleic acids, for binding to complementary mRNAs, and cell penetrating peptides (CPPs), to gain cell entry, along with fluorescent labels and sites for radiolabeling, were prepared by a series of robust, efficient, and versatile synthetic steps that proceeded from monomers to polymers to functional nanoparticles. Amphiphilic block graft copolymers having combinations of methoxy- and thioacetyl-terminated poly(ethylene glycol) (PEG) and DOTA-lysine units grafted from the backbone of poly(acrylic acid) (PAA) and extending with a backbone segment of poly(octadecyl acrylate-co-decyl acrylate) (P(ODA-co-DA)) were prepared by a combination of reversible addition-fragmentation chain transfer (RAFT) polymerization and chemical modification reactions, which were then used as the building blocks for the formation of well-defined SCKs decorated with reactive thiols accessible to the surface. Fluorescent labeling with Alexa Fluor 633 hydrazide was then accomplished by amidation with residual acrylic acid residues within the SCK shells. Finally, the PNAs and CPP units were covalently conjugated to the SCKs via Michael addition of thiols on the SCKs to maleimide units on the termini of PNAs and CPPs. Confirmation of the ability of the PNAs to bind selectively to the target iNOS mRNAs when tethered to the SCK nanoparticles was determined by in vitro competition experiments. When attached to the SCKs having a hydrodynamic diameter of 60 ± 16 nm, the K(d) values of the PNAs were ca. an order of magnitude greater than the free PNAs, while the mismatched PNA showed no significant binding.
Figures


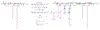

Similar articles
-
Thiol-functionalized shell crosslinked knedel-like (SCK) nanoparticles: A versatile entry for their conjugation with biomacromolecules.Tetrahedron. 2008 Sep 1;64(36):8543-8552. doi: 10.1016/j.tet.2008.04.104. Tetrahedron. 2008. PMID: 19727320 Free PMC article.
-
Construction of thermoresponsive SCKs through tuning the crystalline melting point of the core domain.Soft Matter. 2008 Mar 20;4(4):849-858. doi: 10.1039/b719314a. Soft Matter. 2008. PMID: 32907191
-
Antigen-decorated shell cross-linked nanoparticles: synthesis, characterization, and antibody interactions.Bioconjug Chem. 2005 Sep-Oct;16(5):1246-56. doi: 10.1021/bc0501505. Bioconjug Chem. 2005. PMID: 16173805
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
-
Molecular self-assembly using peptide nucleic acids.Biopolymers. 2017 Jan;108(1):10.1002/bip.22930. doi: 10.1002/bip.22930. Biopolymers. 2017. PMID: 27486924 Free PMC article. Review.
Cited by
-
Degradable cationic shell cross-linked knedel-like nanoparticles: synthesis, degradation, nucleic acid binding, and in vitro evaluation.Biomacromolecules. 2013 Apr 8;14(4):1018-27. doi: 10.1021/bm3018774. Epub 2013 Mar 19. Biomacromolecules. 2013. PMID: 23510389 Free PMC article.
-
Hierarchically assembled theranostic nanostructures for siRNA delivery and imaging applications.J Am Chem Soc. 2012 Oct 24;134(42):17362-5. doi: 10.1021/ja306616n. Epub 2012 Oct 10. J Am Chem Soc. 2012. PMID: 23050597 Free PMC article.
-
Single-step grafting of aminooxy-peptides to hyaluronan: a simple approach to multifunctional therapeutics for experimental autoimmune encephalomyelitis.J Control Release. 2013 Jun 28;168(3):334-40. doi: 10.1016/j.jconrel.2013.03.015. Epub 2013 Mar 27. J Control Release. 2013. PMID: 23541930 Free PMC article.
-
Imaging mRNA Expression in Live Cells via PNA·DNA Strand Displacement-Activated Probes.J Nucleic Acids. 2012;2012:962652. doi: 10.1155/2012/962652. Epub 2012 Sep 26. J Nucleic Acids. 2012. PMID: 23056921 Free PMC article.
-
Antisense peptide nucleic acid-functionalized cationic nanocomplex for in vivo mRNA detection.Interface Focus. 2013 Jun 6;3(3):20120059. doi: 10.1098/rsfs.2012.0059. Interface Focus. 2013. PMID: 24427537 Free PMC article.
References
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and Outcomes of Acute Lung Injury. New England Journal of Medicine. 2005;353:1685–1693. - PubMed
-
- Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine & growth factor reviews. 2003;14:523–535. - PubMed
-
- Hemmrich K, Kröncke KD, Suschek CV, Kolb-Bachofen V. What sense lies in antisense inhibition of inducible nitric oxide synthase expression? Nitric Oxide. 2005;12:183–199. - PubMed
-
- Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, Hudson LD, Matalon S, Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:503–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

