Synthesis and anti-HSV-1 evaluation of new 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines
- PMID: 22373524
- PMCID: PMC3342845
- DOI: 10.1186/2191-2858-2-3
Synthesis and anti-HSV-1 evaluation of new 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines
Abstract
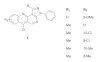
Background: Herpes simplex virus type-1 (HSV-1) is the primary cause of facial lesions (mouth, lips, and eyes) in humans. The widespread use of acyclovir and nucleoside analogues has led to emergence of HSV strains that are resistant to these drugs. Recently, non-nucleoside anti-HSV compounds have received considerable attention. 1,6-Naphthyridines are a class of heterocyclic compounds that exhibit a broad spectrum of biological activities such as inhibitor of HIV-1 integrase, HCMV, FGF receptor-1 tyrosine kinase, and the enzyme acetylcholinesterase. We previously reported the synthesis, SAR studies, and evaluation anti-HSV-1 activity of 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines. In the course of our search for new 1,6-naphthyridines derivatives with potential activity against HSV-1, we have synthesized and evaluated new 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines (1a-k) and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines (2a-c).
Results: A known synthetic approach was used for preparing new 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines (1a-k) and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines (2a-c), starting from ethyl 4-chloro-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate (7). All compounds were identified by FTIR, 1H NMR, and mass spectrometry. The antiviral effect on HSV-1 virus replication was determined.
Conclusions: The compounds 1d, 1f, 1g, and 1h exhibited the highest anti-HSV-1 activity. In general, 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines were more effective inhibitors than their corresponding 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines. The compound 1h reduced the virus yield in 91% at 50 μM and exhibited a low cytotoxicity (CC50 600 μM).
Figures

Similar articles
-
SAR of a series of anti-HSV-1 acridone derivatives, and a rational acridone-based design of a new anti-HSV-1 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine series.Bioorg Med Chem. 2008 Jan 1;16(1):313-21. doi: 10.1016/j.bmc.2007.09.032. Epub 2007 Sep 22. Bioorg Med Chem. 2008. PMID: 17937990
-
Synthesis and Neurotropic Activity of New 5-Piperazinopyrazolo[3,4-c]-2,7-naphthyridines and Isoxazolo[5,4-c]-2,7-naphthyridines.Pharmaceuticals (Basel). 2025 Apr 19;18(4):597. doi: 10.3390/ph18040597. Pharmaceuticals (Basel). 2025. PMID: 40284032 Free PMC article.
-
An efficient Synthesis and Photophysical Properties of 1H-pyrazolo-[3,4-b]pyridine and pyrazolo[3,4-b][1,8]naphthyridines.J Fluoresc. 2015 Nov;25(6):1549-57. doi: 10.1007/s10895-015-1674-2. Epub 2015 Sep 28. J Fluoresc. 2015. PMID: 26411800
-
Naphthyridines with Antiviral Activity - A Review.Med Chem. 2017;13(5):430-438. doi: 10.2174/1573406412666161228112127. Med Chem. 2017. PMID: 28031019 Review.
-
[Studies on the syntheses and reaction of nitrogen-containing heterocyclic compounds centered the naphthyridines].Yakugaku Zasshi. 2000 Feb;120(2):206-23. doi: 10.1248/yakushi1947.120.2_206. Yakugaku Zasshi. 2000. PMID: 10689967 Review. Japanese.
Cited by
-
Anti-HSV-1 agents: an update.Front Pharmacol. 2025 Jan 21;15:1451083. doi: 10.3389/fphar.2024.1451083. eCollection 2024. Front Pharmacol. 2025. PMID: 39931518 Free PMC article. Review.
-
Update on emerging antivirals for the management of herpes simplex virus infections: a patenting perspective.Recent Pat Antiinfect Drug Discov. 2013 Apr;8(1):55-67. doi: 10.2174/1574891x11308010011. Recent Pat Antiinfect Drug Discov. 2013. PMID: 23331181 Free PMC article. Review.
-
The Role of Pyrazolopyridine Derivatives on Different Steps of Herpes Simplex Virus Type-1 In Vitro Replicative Cycle.Int J Mol Sci. 2022 Jul 23;23(15):8135. doi: 10.3390/ijms23158135. Int J Mol Sci. 2022. PMID: 35897709 Free PMC article.
-
Recent advances on heterocyclic compounds with antiviral properties.Chem Heterocycl Compd (N Y). 2021;57(4):410-416. doi: 10.1007/s10593-021-02917-3. Epub 2021 May 12. Chem Heterocycl Compd (N Y). 2021. PMID: 33994556 Free PMC article.
References
-
- James SH, Kimberlin DW, Whitley RJ. Antiviral therapy for herpesvirus central nervous system infections: neonatal herpes simplex virus infection, herpes simplex encephalitis, and congenital cytomegalovirus infection. Antiviral Res. 2009;83:207–213. doi: 10.1016/j.antiviral.2009.04.010. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

