Transient overexpression of cyclin D2/CDK4/GLP1 genes induces proliferation and differentiation of adult pancreatic progenitors and mediates islet regeneration
- PMID: 22373529
- PMCID: PMC3318104
- DOI: 10.4161/cc.11.4.19120
Transient overexpression of cyclin D2/CDK4/GLP1 genes induces proliferation and differentiation of adult pancreatic progenitors and mediates islet regeneration
Abstract
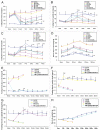
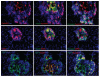
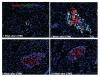
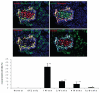
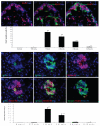
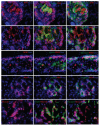
The molecular mechanism of β-cell regeneration remains poorly understood. Cyclin D2/CDK4 expresses in normal β cells and maintains adult β-cell growth. We hypothesized that gene therapy with cyclin D2/CDK4/GLP-1 plasmids targeted to the pancreas of STZ-treated rats by ultrasound-targeted microbubble destruction (UTMD) would force cell cycle re-entry of residual G(0)-phase islet cells into G(1)/S phase to regenerate β cells. A single UTMD treatment induced β-cell regeneration with reversal of diabetes for 6 mo without evidence of toxicity. We observed that this β-cell regeneration was not mediated by self-replication of pre-existing β cells. Instead, cyclin D2/CDK4/GLP-1 initiated robust proliferation of adult pancreatic progenitor cells that exist within islets and terminally differentiate to mature islets with β cells and α cells.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
