PKC phosphorylates GluA1-Ser831 to enhance AMPA receptor conductance
- PMID: 22373567
- PMCID: PMC3367675
- DOI: 10.4161/chan.18648
PKC phosphorylates GluA1-Ser831 to enhance AMPA receptor conductance
Abstract
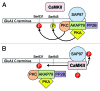
AMPA receptors mediate fast excitatory synaptic transmission in the brain, and are dynamically regulated by phosphorylation of multiple residues within the C-terminal domain. CaMKII phosphorylates Ser831 within the AMPA receptor GluA1 subunit to increase single channel conductance, and biochemical studies show that PKC can also phosphorylate this residue. In light of the discovery of additional PKC phosphorylation sites within the GluA1 C-terminus, it remains unclear whether PKC phosphorylation of Ser831 increases GluA1 conductance in intact receptors. Here, we report that the purified, catalytic subunit of PKC significantly increases the conductance of wild-type GluA1 AMPA receptors expressed in the presence of stargazin in HEK293T cells. Furthermore, the mutation GluA1-S831A blocks the functional effect of PKC. These findings suggest that GluA1 AMPA receptor conductance can be increased by activated CaMKII or PKC, and that phosphorylation at this site provides a mechanism for channel modulation via a variety of protein signaling cascades.
Keywords: AMPA receptor; GluA1; GluR1; PKC; phosphorylation.
Figures

Comment on
- Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, et al. Mechanism of Ca2+/ calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci. 2011;14:727–35. doi: 10.1038/nn.2804. doi: 10.1038/nn.2804
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
