A novel "molecular tweezer" inhibitor of α-synuclein neurotoxicity in vitro and in vivo
- PMID: 22373667
- PMCID: PMC3337029
- DOI: 10.1007/s13311-012-0105-1
A novel "molecular tweezer" inhibitor of α-synuclein neurotoxicity in vitro and in vivo
Erratum in
- Neurotherapeutics. 2012 Apr;9(2):486. Lakshmanan, Ravi [corrected to Lakshmanan, Rajeswari]
Abstract
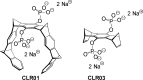
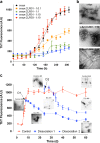
Aggregation of α-synuclein (α-syn) is implicated as being causative in the pathogenesis of Parkinson's disease, multiple system atrophy, and dementia with Lewy bodies. Despite several therapies that improve symptoms in these disorders, none slow disease progression. Recently, a novel "molecular tweezer" (MT) termed CLR01 has been described as a potent inhibitor of assembly and toxicity of multiple amyloidogenic proteins. Here we investigated the ability of CLR01 to inhibit assembly and toxicity of α-syn. In vitro, CLR01 inhibited the assembly of α-syn into β-sheet-rich fibrils and caused disaggregation of pre-formed fibrils, as determined by thioflavin T fluorescence and electron microscopy. α-Syn toxicity was studied in cell cultures and was completely mitigated by CLR01 when α-syn was expressed endogenously or added exogenously. To determine if CLR01 was also protective in vivo, we used a novel zebrafish model of α-syn toxicity (α-syn-ZF), which expresses human, wild-type α-syn in neurons. α-Syn-ZF embryos developed severe deformities due to neuronal apoptosis and most of them died within 48 to 72 h. CLR01 added to the water significantly improved zebrafish phenotype and survival, suppressed α-syn aggregation in neurons, and reduced α-syn-induced apoptosis. α-Syn expression was found to inhibit the ubiquitin proteasome system in α-syn-ZF neurons, resulting in further accumulation of α-syn. Treatment with CLR01 almost completely mitigated the proteasome inhibition. The data suggest that CLR01 is a promising therapeutic agent for the treatment of Parkinson's disease and other synucleinopathies.
Figures




References
-
- Nussbaum, R.L. and M.H. Polymeropoulos, Genetics of Parkinson's disease. Hum Mol Genet, 1997. 6(10): p. 1687–91. - PubMed
-
- Kruger, R., Kuhn, W, Muller, T, et al., Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat Genet, 1998. 18(2): p. 106–8. - PubMed
-
- Trojanowski, J.Q., Goedert, M., Iwatsubo, T., and Lee, V.M. Fatal attractions: abnormal protein aggregation and neuron death in Parkinson's disease and Lewy body dementia. Cell Death Differ, 1998. 5(10): p. 832–7. - PubMed
-
- Spillantini, M.G., Schmidt, M.L., Lee, V.M., Trojanowski, J.Q., Jakes, R., and Goedert, M. α-synuclein in Lewy bodies. Nature, 1997. 388(6645): p. 839–40. - PubMed
-
- Farrer, M., Kachergus, J., Forno, L., et al., Comparison of kindreds with parkinsonism and α-synuclein genomic multiplications. Ann Neurol, 2004. 55(2): p. 174–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

