Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer
- PMID: 22374460
- PMCID: PMC3304403
- DOI: 10.1038/bjc.2012.18
Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer
Abstract
Background: New therapeutic options for metastatic pancreatic cancer are urgently needed. In pancreatic cancer, overexpression of the epidermal growth factor receptor 2 (HER2) has been reported in up to 45%. This multicentre phase II study investigated the efficacy and toxicity of the HER2 antibody trastuzumab combined with capecitabine in the patients with pancreatic cancer and HER2 overexpression.
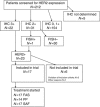
Methods: Primary endpoint was progression-free survival (PFS) after 12 weeks. A total of 212 patients were screened for HER2 expression.
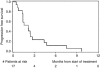
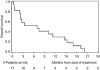
Results: Immunohistochemical (IHC) HER2 expression was: 83 (40%) grade 0, 71 (34%) grade 1, 31 (15%) grade 2, 22 (11%) grade 3. A total of 17 patients with IHC +3 HER2 expression or gene amplification could be assessed for the treatment response. Grade 3/4 treatment toxicities were: each 7% leucopenia, diarrhoea, nausea and hand-foot syndrome. Progression-free survival after 12 weeks was 23.5%, median overall survival (OS) 6.9 months.
Conclusion: This study demonstrates +3 HER2 expression or gene amplification in 11% of patients. Contrary to breast and gastric cancer, only 7 out of 11 (64%) patients with IHC +3 HER2 expression showed gene amplification. Although the therapy was well tolerated, PFS and OS did not perform favourably compared with standard chemotherapy. Together, we do not recommend further evaluation of anti-HER2 treatment in patients with metastatic pancreatic cancer.
Figures
Similar articles
-
A prospective, non-randomized phase II trial of Trastuzumab and Capecitabine in patients with HER2 expressing metastasized pancreatic cancer.BMC Surg. 2009 Jan 8;9:1. doi: 10.1186/1471-2482-9-1. BMC Surg. 2009. PMID: 19133157 Free PMC article.
-
Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer.Eur J Cancer. 2015 Mar;51(4):482-488. doi: 10.1016/j.ejca.2014.12.015. Epub 2015 Feb 3. Eur J Cancer. 2015. PMID: 25661103 Clinical Trial.
-
Lapatinib versus lapatinib plus capecitabine as second-line treatment in human epidermal growth factor receptor 2-amplified metastatic gastro-oesophageal cancer: a randomised phase II trial of the Arbeitsgemeinschaft Internistische Onkologie.Eur J Cancer. 2015 Mar;51(5):569-76. doi: 10.1016/j.ejca.2015.01.059. Epub 2015 Feb 16. Eur J Cancer. 2015. PMID: 25694417 Clinical Trial.
-
[HER2 and gastric cancer: a novel therapeutic target for trastuzumab].Bull Cancer. 2010 Dec;97(12):1429-40. doi: 10.1684/bdc.2010.1224. Bull Cancer. 2010. PMID: 21134821 Review. French.
-
Trastuzumab for the treatment of HER2-positive metastatic adenocarcinoma of the stomach or gastro-oesophageal junction.Health Technol Assess. 2011 May;15 Suppl 1:33-42. doi: 10.3310/hta15suppl1/04. Health Technol Assess. 2011. PMID: 21609651 Review.
Cited by
-
HDACs/mTOR inhibitor synergizes with pyrotinib in HER2-positive pancreatic cancer through degradation of mutant P53.Cancer Cell Int. 2022 Dec 1;22(1):380. doi: 10.1186/s12935-022-02807-4. Cancer Cell Int. 2022. PMID: 36457011 Free PMC article.
-
HER2 aberrations and heterogeneity in cancers of the digestive system: Implications for pathologists and gastroenterologists.World J Gastroenterol. 2016 Sep 21;22(35):7926-37. doi: 10.3748/wjg.v22.i35.7926. World J Gastroenterol. 2016. PMID: 27672288 Free PMC article. Review.
-
Pancreatic cancer from bench to bedside: molecular pathways and treatment options.Ann Transl Med. 2016 May;4(9):165. doi: 10.21037/atm.2016.05.11. Ann Transl Med. 2016. PMID: 27275478 Free PMC article. Review.
-
Anti-CD137 monoclonal antibody enhances trastuzumab-induced, natural killer cell-mediated cytotoxicity against pancreatic cancer cell lines with low human epidermal growth factor-like receptor 2 expression.PLoS One. 2018 Dec 31;13(12):e0200664. doi: 10.1371/journal.pone.0200664. eCollection 2018. PLoS One. 2018. PMID: 30596643 Free PMC article.
-
Current therapeutic strategies for advanced pancreatic cancer: A review for clinicians.World J Clin Oncol. 2016 Feb 10;7(1):27-43. doi: 10.5306/wjco.v7.i1.27. World J Clin Oncol. 2016. PMID: 26862489 Free PMC article. Review.
References
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK, ToGA Trial Investigators (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376: 687–697 - PubMed
-
- Bednar F, Simeone DM (2011) Pancreatic cancer stem cell biology and its therapeutic implications. J Gastroenterol 46: 1345–1352 - PubMed
-
- Buechler P, Reber HA, Buechler MC, Roth MA, Buechler MW, Friess H, Isacoff WH, Hines OJ (2001) Therapy for pancreatic cancer with a recombinant humanized anti HER2 antibody (herceptin). J Gastrointest Surg 5: 139–146 - PubMed
-
- Buechler P, Reber HA, Eibl G, Roth MA, Buechler MW, Friess H, Isacoff WH, Hines OJ (2005) Combination therapy for advanced pancreatic cancer using Herceptin plus chemotherapy. Int J Oncol 27: 1125–1130 - PubMed
-
- Burris H, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous