Neutrophil protease inhibition reduces neuromyelitis optica-immunoglobulin G-induced damage in mouse brain
- PMID: 22374891
- PMCID: PMC3643520
- DOI: 10.1002/ana.22686
Neutrophil protease inhibition reduces neuromyelitis optica-immunoglobulin G-induced damage in mouse brain
Abstract
Objective: Neuromyelitis optica (NMO) is an inflammatory demyelinating disease of the central nervous system associated with pathogenic autoantibodies against the astrocyte water channel protein aquaporin-4 (AQP4). The presence of neutrophils is a characteristic feature in NMO lesions in humans. Neutrophils are not generally found in multiple sclerosis lesions. We evaluated the role of neutrophils in a mouse NMO model.
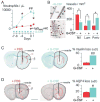
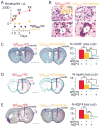
Methods: NMO lesions were produced in mice by intracerebral injection of immunoglobulin G (IgG) isolated from NMO patient serum and human complement. We previously reported that this mouse model produces the characteristic histological features of NMO, including perivascular complement activation, inflammatory cell infiltration, and loss of myelin, AQP4, and glial fibrillary acidic protein. Lesions are absent when AQP4 null mice are used or when IgG from non-NMO patients is injected.
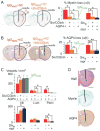
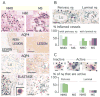
Results: We found remarkably reduced neuroinflammation, myelin loss, and AQP4 loss in brains of neutropenic mice at 24 hours and 7 days, and increased severity of NMO lesions in mice made neutrophilic by granulocyte colony stimulating factor. NMO lesions were greatly reduced by intracerebral administration of the neutrophil protease inhibitors Sivelestat and cathepsin G inhibitor I or by intraperitoneal injection of Sivelestat alone. Immunostaining of human NMO lesions for neutrophil elastase revealed many degranulating perivascular neutrophils, with no equivalent perivascular neutrophils in human multiple sclerosis lesions.
Interpretation: Our data implicate a central role of neutrophils in the pathogenesis of early NMO lesions and suggest the potential utility of neutrophil protease inhibitors such as Sivelestat in NMO therapy.
Copyright © 2012 American Neurological Association.
Conflict of interest statement
A.S.V.: grants: EY13574 and DK35124 from the National Institutes of Health, and Guthy-Jackson Charitable Foundation. A.V.: grant: Oxford NIHR Biomedical Research Centre. M.C.P.: grant: Guthy-Jackson Charitable Foundation. P.W.: grant: Oxford NIHR Biomedical Research Centre; employment: University of Oxford; patents: patents for diagnostic assays for Lgi1 and Caspar2.
Figures

References
-
- Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. - PubMed
-
- Jarius S, Paul F, Franciotta D, et al. Mechanisms of disease: aquaporin- 4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008;4:202–214. - PubMed
-
- Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol. 2010;6:383–392. - PubMed
-
- Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

