Carnosol, a constituent of Zyflamend, inhibits aryl hydrocarbon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and mutagenesis
- PMID: 22374940
- PMCID: PMC3324618
- DOI: 10.1158/1940-6207.CAPR-12-0002
Carnosol, a constituent of Zyflamend, inhibits aryl hydrocarbon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and mutagenesis
Retraction in
-
Retraction: Carnosol, a Constituent of Zyflamend, Inhibits Aryl Hydrocarbon Receptor-Mediated Activation of CYP1A1 and CYP1B1 Transcription and Mutagenesis.Cancer Prev Res (Phila). 2022 Jun 2;15(6):412. doi: 10.1158/1940-6207.CAPR-22-0203. Cancer Prev Res (Phila). 2022. PMID: 35652229 Free PMC article. No abstract available.
Abstract
The aryl hydrocarbon receptor (AhR), a ligand-activated member of the basic helix-loop-helix family of transcription factors, plays a significant role in polycyclic aromatic hydrocarbon (PAH)-induced carcinogenesis. In the upper aerodigestive tract of humans, tobacco smoke, a source of PAHs, activates the AhR leading to increased expression of CYP1A1 and CYP1B1, which encode proteins that convert PAHs to genotoxic metabolites. Inhibitors of Hsp90 ATPase cause a rapid decrease in levels of AhR, an Hsp90 client protein, and thereby block PAH-mediated induction of CYP1A1 and CYP1B1. The main objective of this study was to determine whether Zyflamend, a polyherbal preparation, suppressed PAH-mediated induction of CYP1A1 and CYP1B1 and inhibited DNA adduct formation and mutagenesis. We also investigated whether carnosol, one of multiple phenolic antioxidants in Zyflamend, had similar inhibitory effects. Treatment of cell lines derived from oral leukoplakia (MSK-Leuk1) and skin (HaCaT) with benzo[a]pyrene (B[a]P), a prototypic PAH, induced CYP1A1 and CYP1B1 transcription, resulting in enhanced levels of message and protein. Both Zyflamend and carnosol suppressed these effects of B[a]P. Notably, both Zyflamend and carnosol inhibited Hsp90 ATPase activity and caused a rapid reduction in AhR levels. The formation of B[a]P-induced DNA adducts and mutagenesis was also inhibited by Zyflamend and carnosol. Collectively, these results show that Zyflamend and carnosol inhibit Hsp90 ATPase leading to reduced levels of AhR, suppression of B[a]P-mediated induction of CYP1A1 and CYP1B1, and inhibition of mutagenesis. Carnosol-mediated inhibition of Hsp90 ATPase activity can help explain the chemopreventive activity of herbs such as Rosemary, which contain this phenolic antioxidant.
2012 AACR
Conflict of interest statement
Andrew J. Dannenberg is a member of the scientific advisory board of Tragara Pharmaceuticals, Inc.
Figures
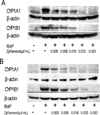
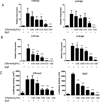
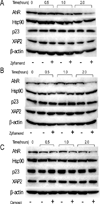
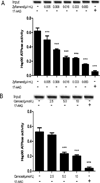

Comment in
-
Findings of Research Misconduct.Fed Regist. 2023 Sep 13;88(176):62800-62803. Fed Regist. 2023. PMID: 37736072 Free PMC article. No abstract available.
-
Findings of Research Misconduct.Fed Regist. 2023 Sep 13;88(176):62803-62807. Fed Regist. 2023. PMID: 37736073 Free PMC article. No abstract available.
References
-
- Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–561. - PubMed
-
- Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzop-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976;251(16):4936–4946. - PubMed
-
- Bock KW, Kohle C. Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol. 2006;72(4):393–404. - PubMed
-
- Moennikes O, Loeppen S, Buchmann A, Andersson P, Ittrich C, Poellinger L, et al. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res. 2004;64(14):4707–4710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

