Identification of N-methyl-D-aspartic acid (NMDA) receptor subtype-specific binding sites that mediate direct interactions with scaffold protein PSD-95
- PMID: 22375001
- PMCID: PMC3339998
- DOI: 10.1074/jbc.M111.292862
Identification of N-methyl-D-aspartic acid (NMDA) receptor subtype-specific binding sites that mediate direct interactions with scaffold protein PSD-95
Abstract
N-methyl-D-aspartate (NMDA) neurotransmitter receptors and the postsynaptic density-95 (PSD-95) membrane-associated guanylate kinase (MAGUK) family of scaffolding proteins are integral components of post-synaptic macromolecular signaling complexes that serve to propagate glutamate responses intracellularly. Classically, NMDA receptor NR2 subunits associate with PSD-95 MAGUKs via a conserved ES(E/D)V amino acid sequence located at their C termini. We previously challenged this dogma to demonstrate a second non-ES(E/D)V PSD-95-binding site in both NMDA receptor NR2A and NR2B subunits. Here, using a combination of co-immunoprecipitations from transfected mammalian cells, yeast two-hybrid interaction assays, and glutathione S-transferase (GST) pulldown assays, we show that NR2A subunits interact directly with PSD-95 via the C-terminal ESDV motif and additionally via an Src homology 3 domain-binding motif that associates with the Src homology 3 domain of PSD-95. Peptide inhibition of co-immunoprecipitations of NR2A and PSD-95 demonstrates that both the ESDV and non-ESDV sites are required for association in native brain tissue. Furthermore, we refine the non-ESDV site within NR2B to residues 1149-1157. These findings provide a molecular basis for the differential association of NMDA receptor subtypes with PSD-95 MAGUK scaffold proteins. These selective interactions may contribute to the organization, lateral mobility, and ultimately the function of NMDA receptor subtypes at synapses. Furthermore, they provide a more general molecular mechanism by which the scaffold, PSD-95, may discriminate between potential interacting partner proteins.
Figures


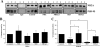



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

