A unique series of reversibly switchable fluorescent proteins with beneficial properties for various applications
- PMID: 22375034
- PMCID: PMC3311367
- DOI: 10.1073/pnas.1113770109
A unique series of reversibly switchable fluorescent proteins with beneficial properties for various applications
Abstract
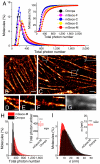
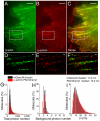
Reversibly switchable fluorescent proteins (RSFPs) have attracted widespread interest for emerging techniques including repeated tracking of protein behavior and superresolution microscopy. Among the limited number of RSFPs available, only Dronpa is widely employed for most cell biology applications due to its monomeric and other favorable photochemical properties. Here we developed a series of monomeric green RSFPs with beneficial optical characteristics such as high photon output per switch, high photostability, a broad range of switching rate, and pH-dependence, which make them potentially useful for various applications. One member of this series, mGeos-M, exhibits the highest photon budget and localization precision potential among all green RSFPs. We propose mGeos-M as a candidate to replace Dronpa for applications such as dynamic tracking, dual-color superresolution imaging, and optical lock-in detection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Photoswitchable fluorescent proteins enable monochromatic multilabel imaging and dual color fluorescence nanoscopy.Nat Biotechnol. 2008 Sep;26(9):1035-40. doi: 10.1038/nbt.1493. Nat Biotechnol. 2008. PMID: 18724362
-
Highly photostable, reversibly photoswitchable fluorescent protein with high contrast ratio for live-cell superresolution microscopy.Proc Natl Acad Sci U S A. 2016 Sep 13;113(37):10364-9. doi: 10.1073/pnas.1611038113. Epub 2016 Aug 25. Proc Natl Acad Sci U S A. 2016. PMID: 27562163 Free PMC article.
-
Dual channel RESOLFT nanoscopy by using fluorescent state kinetics.Nano Lett. 2015 Jan 14;15(1):103-6. doi: 10.1021/nl503058k. Epub 2014 Dec 2. Nano Lett. 2015. PMID: 25423166
-
Structural basis of photoswitching in fluorescent proteins.Methods Mol Biol. 2014;1148:177-202. doi: 10.1007/978-1-4939-0470-9_12. Methods Mol Biol. 2014. PMID: 24718802 Review.
-
Recent advances using green and red fluorescent protein variants.Appl Microbiol Biotechnol. 2007 Nov;77(1):1-12. doi: 10.1007/s00253-007-1131-5. Epub 2007 Aug 18. Appl Microbiol Biotechnol. 2007. PMID: 17704916 Review.
Cited by
-
Room-temperature photo-induced martensitic transformation in a protein crystal.IUCrJ. 2019 May 22;6(Pt 4):619-629. doi: 10.1107/S2052252519005761. eCollection 2019 Jul 1. IUCrJ. 2019. Retraction in: IUCrJ. 2019 Jun 06;6(Pt 4):781. doi: 10.1107/S2052252519007851. PMID: 31316806 Free PMC article. Retracted.
-
Chromophore chemistry of fluorescent proteins controlled by light.Curr Opin Chem Biol. 2014 Jun;20:60-8. doi: 10.1016/j.cbpa.2014.04.010. Epub 2014 May 13. Curr Opin Chem Biol. 2014. PMID: 24819887 Free PMC article. Review.
-
Rational design of ultrastable and reversibly photoswitchable fluorescent proteins for super-resolution imaging of the bacterial periplasm.Sci Rep. 2016 Jan 6;6:18459. doi: 10.1038/srep18459. Sci Rep. 2016. PMID: 26732634 Free PMC article.
-
Choosing proper fluorescent dyes, proteins, and imaging techniques to study mitochondrial dynamics in mammalian cells.Biophys Rep. 2017;3(4):64-72. doi: 10.1007/s41048-017-0037-8. Epub 2017 Mar 24. Biophys Rep. 2017. PMID: 29238743 Free PMC article.
-
Three-dimensional super-resolution protein localization correlated with vitrified cellular context.Sci Rep. 2015 Oct 14;5:13017. doi: 10.1038/srep13017. Sci Rep. 2015. PMID: 26462878 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

