Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trials
- PMID: 22375098
- PMCID: PMC3287409
- DOI: 10.2147/DMSO.S28387
Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trials
Abstract
Background: Exenatide twice daily is a first-in-class glucagon-like peptide receptor agonist approved for the treatment of type 2 diabetes. The objective of this analysis was to evaluate the safety profile of exenatide twice daily and to compare its profile with that of a pooled comparator (placebo and insulin) in patients with type 2 diabetes.
Methods: Data from 19 completed, randomized, controlled clinical trials of exenatide twice daily (5 μg and 10 μg) were pooled and analyzed; the pooled data included 5594 intent-to-treat patients who were followed for 12-52 weeks. Incidence rates, exposure-adjusted incidence rates, and 95% confidence intervals around risk differences between groups were calculated.
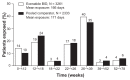
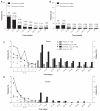
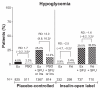
Results: Baseline demographics and exposure time were comparable between groups (exenatide, N = 3261; pooled comparator, N = 2333; mean exposure time 166-171 days). Transient, mild- to-moderate nausea was the most frequent adverse event with exenatide (36.9% versus 8.3% in the pooled comparator). The incidence of hypoglycemia (minor or major) with concomitant sulfonylurea (exenatide 26.5%, pooled comparator 20.7%) was higher than that without sulfonylurea (exenatide 3.1%, pooled comparator 2.7%) in all groups. Serious adverse events, discontinuations due to serious adverse events, and deaths were reported with similar frequency in the exenatide and pooled comparator groups. Composite exposure-adjusted incidence rates were not statistically different between groups for pancreatitis, renal impairment, or major adverse cardiac events; there was a difference in incidence rates for benign thyroid neoplasm (0.3% versus 0%).
Conclusion: Overall, this analysis, representing over 1500 patient-years of exposure, demonstrated that exenatide twice daily was safe and generally well tolerated in patients with type 2 diabetes. The incidence of most adverse events, including serious adverse events, was similar in both exenatide-treated and comparator-treated patients. The most distinct differences between groups were in gastrointestinal-related adverse events, which is consistent with other therapies within the glucagon-like peptide class.
Keywords: adverse events; exenatide; risk difference; safety.
Figures


Similar articles
-
Safety and tolerability of exenatide once weekly in patients with type 2 diabetes: an integrated analysis of 4,328 patients.Diabetes Metab Syndr Obes. 2015 May 18;8:241-53. doi: 10.2147/DMSO.S77290. eCollection 2015. Diabetes Metab Syndr Obes. 2015. PMID: 26056482 Free PMC article.
-
Pancreatitis Incidence in the Exenatide BID, Exenatide QW, and Exenatide QW Suspension Development Programs: Pooled Analysis of 35 Clinical Trials.Diabetes Ther. 2019 Aug;10(4):1249-1270. doi: 10.1007/s13300-019-0627-1. Epub 2019 May 10. Diabetes Ther. 2019. PMID: 31077072 Free PMC article.
-
Safety and Tolerability of Linagliptin in Patients With Type 2 Diabetes: A Comprehensive Pooled Analysis of 22 Placebo-controlled Studies.Clin Ther. 2014 Aug 1;36(8):1130-46. doi: 10.1016/j.clinthera.2014.06.008. Epub 2014 Jul 8. Clin Ther. 2014. PMID: 25015594
-
Antibiotics for induction and maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2019 Feb 7;2(2):CD012730. doi: 10.1002/14651858.CD012730.pub2. Cochrane Database Syst Rev. 2019. PMID: 30731030 Free PMC article.
-
Exenatide extended-release: a review of its use in type 2 diabetes mellitus.Drugs. 2012 Aug 20;72(12):1679-707. doi: 10.2165/11209750-000000000-00000. Drugs. 2012. PMID: 22867046 Review.
Cited by
-
Type 2 diabetes mellitus and the cardiometabolic syndrome: impact of incretin-based therapies.Diabetes Metab Syndr Obes. 2010 Jul 9;3:227-42. doi: 10.2147/dmsott.s11389. Diabetes Metab Syndr Obes. 2010. PMID: 21437091 Free PMC article.
-
Effectiveness and safety of exenatide in Korean patients with type 2 diabetes inadequately controlled with oral hypoglycemic agents: an observational study in a real clinical practice.BMC Endocr Disord. 2017 Oct 25;17(1):68. doi: 10.1186/s12902-017-0220-4. BMC Endocr Disord. 2017. PMID: 29065865 Free PMC article.
-
Expression and Characterization of a Potent Long-Acting GLP-1 Receptor Agonist, GLP-1-IgG2σ-Fc.PLoS One. 2016 May 27;11(5):e0156449. doi: 10.1371/journal.pone.0156449. eCollection 2016. PLoS One. 2016. PMID: 27232339 Free PMC article.
-
Pharmacologic Therapy of Diabetes and Overall Cancer Risk and Mortality: A Meta-Analysis of 265 Studies.Sci Rep. 2015 Jun 15;5:10147. doi: 10.1038/srep10147. Sci Rep. 2015. PMID: 26076034 Free PMC article.
-
Safety and Tolerability of Glucagon-Like Peptide-1 Receptor Agonists Utilizing Data from the Exenatide Clinical Trial Development Program.Curr Diab Rep. 2016 May;16(5):44. doi: 10.1007/s11892-016-0728-4. Curr Diab Rep. 2016. PMID: 27037706 Review.
References
-
- Norris SL, Lee N, Thakurta S, Chan BK. Exenatide efficacy and safety: a systematic review. Diabet Med. 2009;26(9):837–846. - PubMed
-
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628–2635. - PubMed
-
- DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–1100. - PubMed
-
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083–1091. - PubMed
-
- Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143(8):559–569. - PubMed
LinkOut - more resources
Full Text Sources

