Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trials
- PMID: 22375098
- PMCID: PMC3287409
- DOI: 10.2147/DMSO.S28387
Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trials
Abstract
Background: Exenatide twice daily is a first-in-class glucagon-like peptide receptor agonist approved for the treatment of type 2 diabetes. The objective of this analysis was to evaluate the safety profile of exenatide twice daily and to compare its profile with that of a pooled comparator (placebo and insulin) in patients with type 2 diabetes.
Methods: Data from 19 completed, randomized, controlled clinical trials of exenatide twice daily (5 μg and 10 μg) were pooled and analyzed; the pooled data included 5594 intent-to-treat patients who were followed for 12-52 weeks. Incidence rates, exposure-adjusted incidence rates, and 95% confidence intervals around risk differences between groups were calculated.
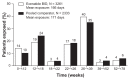
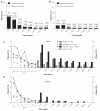
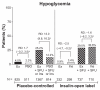
Results: Baseline demographics and exposure time were comparable between groups (exenatide, N = 3261; pooled comparator, N = 2333; mean exposure time 166-171 days). Transient, mild- to-moderate nausea was the most frequent adverse event with exenatide (36.9% versus 8.3% in the pooled comparator). The incidence of hypoglycemia (minor or major) with concomitant sulfonylurea (exenatide 26.5%, pooled comparator 20.7%) was higher than that without sulfonylurea (exenatide 3.1%, pooled comparator 2.7%) in all groups. Serious adverse events, discontinuations due to serious adverse events, and deaths were reported with similar frequency in the exenatide and pooled comparator groups. Composite exposure-adjusted incidence rates were not statistically different between groups for pancreatitis, renal impairment, or major adverse cardiac events; there was a difference in incidence rates for benign thyroid neoplasm (0.3% versus 0%).
Conclusion: Overall, this analysis, representing over 1500 patient-years of exposure, demonstrated that exenatide twice daily was safe and generally well tolerated in patients with type 2 diabetes. The incidence of most adverse events, including serious adverse events, was similar in both exenatide-treated and comparator-treated patients. The most distinct differences between groups were in gastrointestinal-related adverse events, which is consistent with other therapies within the glucagon-like peptide class.
Keywords: adverse events; exenatide; risk difference; safety.
Figures


References
-
- Norris SL, Lee N, Thakurta S, Chan BK. Exenatide efficacy and safety: a systematic review. Diabet Med. 2009;26(9):837–846. - PubMed
-
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628–2635. - PubMed
-
- DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–1100. - PubMed
-
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083–1091. - PubMed
-
- Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143(8):559–569. - PubMed
LinkOut - more resources
Full Text Sources

