Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood
- PMID: 22375940
- PMCID: PMC3380160
- DOI: 10.1111/j.1365-2826.2012.02306.x
Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood
Abstract
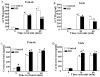
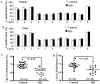
Exposure to stress during early development causes long-lasting alterations in behaviour and hypothalamic pituitary adrenal (HPA) axis activity, including increased expression of corticotrophin-releasing hormone (CRH). To determine whether early-life stress causes epigenetic changes in the CRH promoter leading to increased CRH transcription, 8-week old female and male rats, subjected to maternal deprivation (MD) between days 2 and 13 post-birth, were studied for HPA axis responses to stress and CRH promoter methylation in the hypothalamic paraventricular nucleus (PVN) and central nucleus of the amygdala (CeA). Plasma corticosterone and PVN CRH heteronuclear (hn)RNA responses to acute restraint stress were higher in MD rats of both sexes. DNA methylation analysis of the CRH promoter revealed a significantly lower percentage of methylation in two CpGs preceding (CpG1) and inside (CpG2) the cyclic AMP-response element (CRE) at -230 bp in the CRH promoter in the PVN but not the CeA of MD rats. Gel-shift assays, using nuclear proteins from forskolin-treated hypothalamic 4B cells and CRH promoter CRE oligonucleotides, unmethylated or methylated at CpG1, revealed a strong band that was supershifted by phospho-cAMP response element-binding antibody. This band was 50% weaker using oligonucleotides methylated at CpG2 (intra-CRE), or methylated at both CpG1 and CpG2. These findings demonstrate that HPA axis hypersensitivity caused by neonatal stress causes long-lasting enhanced CRH transcriptional activity in the PVN of both sexes. Hypomethylation of the CRH promoter CRE, a region critical for CRH transcriptional activation, could serve as a mechanism for the increased transcriptional responses to stress observed in MD rats.
Published 2012. This article is a US Government work and is in the public domain in the USA.
Figures
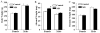




References
-
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–39. - PubMed
-
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4(2):163–72. - PubMed
-
- Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D. The Roscommon Family Study. III. Schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993;50(10):781–8. - PubMed
-
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder. Same genes, (partly) different environments? Arch Gen Psychiatry. 1992;49(9):716–22. - PubMed
-
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Childhood parental loss and adult psychopathology in women. A twin study perspective. Arch Gen Psychiatry. 1992;49(2):109–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

