Horizontal transmission of the symbiotic bacterium Asaia sp. in the leafhopper Scaphoideus titanus Ball (Hemiptera: Cicadellidae)
- PMID: 22376056
- PMCID: PMC3287515
- DOI: 10.1186/1471-2180-12-S1-S4
Horizontal transmission of the symbiotic bacterium Asaia sp. in the leafhopper Scaphoideus titanus Ball (Hemiptera: Cicadellidae)
Abstract
Background: Bacteria of the genus Asaia have been recently recognized as secondary symbionts of different sugar-feeding insects, including the leafhopper Scaphoideus titanus, vector of Flavescence dorée phytoplasmas. Asaia has been shown to be localized in S. titanus gut, salivary glands and gonoducts and to be maternally transmitted to the progeny by an egg smearing mechanism. It is currently not known whether Asaia in S. titanus is transmitted by additional routes. We performed a study to evaluate if Asaia infection is capable of horizontal transmission via co-feeding and venereal routes.
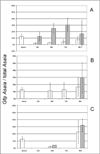
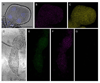
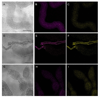
Results: A Gfp-tagged strain of Asaia was provided to S. titanus individuals to trace the transmission pathways of the symbiotic bacterium. Co-feeding trials showed a regular transfer of bacterial cells from donors to recipients, with a peak of frequency after 72 hours of exposure, and with concentrations of the administrated strain growing over time. Venereal transmission experiments were first carried out using infected males paired with uninfected females. In this case, female individuals acquired Gfp-labelled Asaia, with highest infection rates 72-96 hours after mating and with increasing abundance of the tagged symbiont over time. When crosses between infected females and uninfected males were conducted, the occurrence of "female to male" transmission was observed, even though the transfer occurred unevenly.
Conclusions: The data presented demonstrate that the acetic acid bacterial symbiont Asaia is horizontally transmitted among S. titanus individuals both by co-feeding and venereal transmission, providing one of the few direct demonstrations of such a symbiotic transfer in Hemiptera. This study contributes to the understanding of the bacterial ecology in the insect host, and indicates that Asaia evolved multiple pathways for the colonization of S. titanus body.
Figures

Similar articles
-
Asaia, a versatile acetic acid bacterial symbiont, capable of cross-colonizing insects of phylogenetically distant genera and orders.Environ Microbiol. 2009 Dec;11(12):3252-64. doi: 10.1111/j.1462-2920.2009.02048.x. Epub 2009 Sep 4. Environ Microbiol. 2009. PMID: 19735280
-
Multiple symbiosis in the leafhopper Scaphoideus titanus (Hemiptera: Cicadellidae): details of transovarial transmission of Cardinium sp. and yeast-like endosymbionts.Tissue Cell. 2008 Aug;40(4):231-42. doi: 10.1016/j.tice.2007.12.005. Epub 2008 Feb 13. Tissue Cell. 2008. PMID: 18272191
-
A novel Bacteroidetes symbiont is localized in Scaphoideus titanus, the insect vector of Flavescence dorée in Vitis vinifera.Appl Environ Microbiol. 2006 Feb;72(2):1467-75. doi: 10.1128/AEM.72.2.1467-1475.2006. Appl Environ Microbiol. 2006. PMID: 16461701 Free PMC article.
-
Acetic acid bacteria, newly emerging symbionts of insects.Appl Environ Microbiol. 2010 Nov;76(21):6963-70. doi: 10.1128/AEM.01336-10. Epub 2010 Sep 17. Appl Environ Microbiol. 2010. PMID: 20851977 Free PMC article. Review.
-
Bacteria of the genus Asaia: a potential paratransgenic weapon against malaria.Adv Exp Med Biol. 2008;627:49-59. doi: 10.1007/978-0-387-78225-6_4. Adv Exp Med Biol. 2008. PMID: 18510013 Review.
Cited by
-
Microbial symbionts: a resource for the management of insect-related problems.Microb Biotechnol. 2012 May;5(3):307-17. doi: 10.1111/j.1751-7915.2011.00312.x. Epub 2011 Nov 22. Microb Biotechnol. 2012. PMID: 22103294 Free PMC article. Review.
-
Genome Reduction in the Mosquito Symbiont Asaia.Genome Biol Evol. 2019 Jan 1;11(1):1-10. doi: 10.1093/gbe/evy255. Genome Biol Evol. 2019. PMID: 30476071 Free PMC article.
-
Characterization of a Bacterial Symbiont Asaia sp. in the White-Backed Planthopper, Sogatella furcifera, and Its Effects on Host Fitness.Front Microbiol. 2019 Sep 18;10:2179. doi: 10.3389/fmicb.2019.02179. eCollection 2019. Front Microbiol. 2019. PMID: 31620116 Free PMC article.
-
Control of Scaphoideus titanus with Natural Products in Organic Vineyards.Insects. 2017 Dec 16;8(4):129. doi: 10.3390/insects8040129. Insects. 2017. PMID: 29258165 Free PMC article.
-
Microbiome analysis of monarch butterflies reveals effects of development and diet.FEMS Microbiol Ecol. 2024 Nov 23;100(12):fiae143. doi: 10.1093/femsec/fiae143. FEMS Microbiol Ecol. 2024. PMID: 39557647 Free PMC article.
References
-
- Kommanee J, Akaracharanya A, Tanasupawat S, Malimas T, Yukphan P, Nakagawa Y, Yamada Y. Identification of Acetobacter strains isolated in Thailand based on 16S-23S rRNA gene ITS restriction and 16S rRNA gene sequence analyses. Ann Microbiol. 2008;58:319–324. doi: 10.1007/BF03175337. - DOI
-
- Bertaccini A, Duduk B. Phytoplasma and phytoplasma diseases: a review of recent research. Phytopathol Mediter. 2009;48:355–378.
-
- Crotti E, Damiani C, Pajoro M, Gonella E, Rizzi A, Ricci I, Negri I, Scuppa P, Rossi P, Ballarini P, Raddadi N, Marzorati M, Sacchi L, Clementi E, Genchi M, Mandrioli Bandi C, Favia G, Alma A, Daffonchio D. Asaia, a versatile acetic acid bacterial symbiont, capable of cross-colonizing insects of phylogenetically distant genera and orders. Environ Microbiol. 2009;11:3252–3264. doi: 10.1111/j.1462-2920.2009.02048.x. - DOI - PubMed
-
- Damiani C, Ricci I, Crotti E, Rossi P, Rizzi A, Scuppa P, Capone A, Ulissi U, Epis S, Genchi M, Sagnon N, Faye I, Kang A, Chouaia B, Whitehorn C, Moussa GW, Mandrioli M, Esposito F, Sacchi L, Bandi C, Daffonchio D, Favia G. Mosquito-bacteria symbiosis: the case of Anopheles gambiae and Asaia. Microb Ecol. 2010;60:644–54. doi: 10.1007/s00248-010-9704-8. - DOI - PubMed

