Field testing of a multicriteria decision analysis (MCDA) framework for coverage of a screening test for cervical cancer in South Africa
- PMID: 22376143
- PMCID: PMC3330006
- DOI: 10.1186/1478-7547-10-2
Field testing of a multicriteria decision analysis (MCDA) framework for coverage of a screening test for cervical cancer in South Africa
Abstract
Background: Systematic and transparent approaches to priority setting are needed, particularly in low-resource settings, to produce decisions that are sound and acceptable to stakeholders. The EVIDEM framework brings together Health Technology Assessment (HTA) and multi-criteria decision analysis (MCDA) by proposing a comprehensive set of decision criteria together with standardized processes to support decisionmaking. The objective of the study was to field test the framework for decisionmaking on a screening test by a private health plan in South Africa.
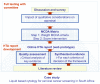
Methods: Liquid-based cytology (LBC) for cervical cancer screening was selected by the health plan for this field test. An HTA report structured by decision criterion (14 criteria organized in the MCDA matrix and 4 contextual criteria) was produced based on a literature review and input from the health plan. During workshop sessions, committee members 1) weighted each MCDA decision criterion to express their individual perspectives, and 2) to appraise LBC, assigned scores to each MCDA criterion on the basis of the by-criterion HTA report.Committee members then considered the potential impacts of four contextual criteria on the use of LBC in the context of their health plan. Feedback on the framework and process was collected through discussion and from a questionnaire.
Results: For 9 of the MCDA matrix decision criteria, 89% or more of committee members thought they should always be considered in decisionmaking. Greatest weights were given to the criteria "Budget impact", "Cost-effectiveness" and "Completeness and consistency of reporting evidence". When appraising LBC for cervical cancer screening, the committee assigned the highest scores to "Relevance and validity of evidence" and "Disease severity". Combination of weights and scores yielded a mean MCDA value estimate of 46% (SD 7%) of the potential maximum value. Overall, the committee felt the framework brought greater clarity to the decisionmaking process and was easily adaptable to different types of health interventions.
Conclusions: The EVIDEM framework was easily adapted to evaluating a screening technology in South Africa, thereby broadening its applicability in healthcare decision making.
Figures



Similar articles
-
Bridging health technology assessment (HTA) with multicriteria decision analyses (MCDA): field testing of the EVIDEM framework for coverage decisions by a public payer in Canada.BMC Health Serv Res. 2011 Nov 30;11:329. doi: 10.1186/1472-6963-11-329. BMC Health Serv Res. 2011. PMID: 22129247 Free PMC article.
-
Multicriteria decision analysis (MCDA) for health technology assessment: the Queensland Health experience.Aust Health Rev. 2019 Oct;43(5):591-599. doi: 10.1071/AH18042. Aust Health Rev. 2019. PMID: 30205873
-
Bridging health technology assessment (HTA) and efficient health care decision making with multicriteria decision analysis (MCDA): applying the EVIDEM framework to medicines appraisal.Med Decis Making. 2012 Mar-Apr;32(2):376-88. doi: 10.1177/0272989X11416870. Epub 2011 Oct 10. Med Decis Making. 2012. PMID: 21987539
-
Evidence and Value: Impact on DEcisionMaking--the EVIDEM framework and potential applications.BMC Health Serv Res. 2008 Dec 22;8:270. doi: 10.1186/1472-6963-8-270. BMC Health Serv Res. 2008. PMID: 19102752 Free PMC article. Review.
-
Multicriteria Decision Analysis to Support Health Technology Assessment Agencies: Benefits, Limitations, and the Way Forward.Value Health. 2019 Nov;22(11):1283-1288. doi: 10.1016/j.jval.2019.06.014. Epub 2019 Oct 16. Value Health. 2019. PMID: 31708065
Cited by
-
Examining the association between oncology drug clinical benefit and the time to public reimbursement.Cancer Med. 2022 Jan;11(2):380-391. doi: 10.1002/cam4.4455. Epub 2021 Dec 1. Cancer Med. 2022. PMID: 34850587 Free PMC article.
-
Incorporating MCDA into HTA: challenges and potential solutions, with a focus on lower income settings.Cost Eff Resour Alloc. 2018 Nov 9;16(Suppl 1):43. doi: 10.1186/s12962-018-0125-8. eCollection 2018. Cost Eff Resour Alloc. 2018. PMID: 30455602 Free PMC article. Review.
-
Balancing costs and benefits at different stages of medical innovation: a systematic review of Multi-criteria decision analysis (MCDA).BMC Health Serv Res. 2015 Jul 9;15:262. doi: 10.1186/s12913-015-0930-0. BMC Health Serv Res. 2015. PMID: 26152122 Free PMC article.
-
Exploring the perspectives and preferences for HTA across German healthcare stakeholders using a multi-criteria assessment of a pulmonary heart sensor as a case study.Health Res Policy Syst. 2015 Apr 28;13:24. doi: 10.1186/s12961-015-0011-1. Health Res Policy Syst. 2015. PMID: 25928535 Free PMC article.
-
Does technique matter; a pilot study exploring weighting techniques for a multi-criteria decision support framework.Cost Eff Resour Alloc. 2014 Nov 18;12:22. doi: 10.1186/1478-7547-12-22. eCollection 2014. Cost Eff Resour Alloc. 2014. PMID: 25904823 Free PMC article.
References
LinkOut - more resources
Full Text Sources

