Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 Immunohistochemistry, Consensus PCR HPV-DNA, and In Situ Hybridization
- PMID: 22376902
- PMCID: PMC3313884
- DOI: 10.1186/1750-9378-7-4
Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 Immunohistochemistry, Consensus PCR HPV-DNA, and In Situ Hybridization
Abstract
Background: Recent emerging evidences identify Human Papillomavirus (HPV) related Head and Neck squamous cell carcinomas (HN-SCCs) as a separate subgroup among Head and Neck Cancers with different epidemiology, histopathological characteristics, therapeutic response to chemo-radiation treatment and clinical outcome. However, there is not a worldwide consensus on the methods to be used in clinical practice. The endpoint of this study was to demonstrate the reliability of a triple method which combines evaluation of: 1. p16 protein expression by immunohistochemistry (p16-IHC); 2. HPV-DNA genotyping by consensus HPV-DNA PCR methods (Consensus PCR); and 3 viral integration into the host by in situ hybridization method (ISH). This triple method has been applied to HN-SCC originated from oral cavity (OSCC) and oropharynx (OPSCC), the two anatomical sites in which high risk (HR) HPVs have been clearly implicated as etiologic factors. Methylation-Specific PCR (MSP) was performed to study inactivation of p16-CDKN2a locus by epigenetic events. Reliability of multiple methods was measured by Kappa statistics.
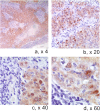
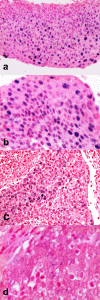
Results: All the HN-SCCs confirmed HPV positive by PCR and/or ISH were also p16 positive by IHC, with the latter showing a very high level of sensitivity as single test (100% in both OSCC and OPSCC) but lower specificity level (74% in OSCC and 93% in OPSCC).Concordance analysis between ISH and Consensus PCR showed a faint agreement in OPSCC (κ = 0.38) and a moderate agreement in OSCC (κ = 0.44). Furthermore, the addition of double positive score (ISHpositive and Consensus PCR positive) increased significantly the specificity of HR-HPV detection on formalin-fixed paraffin embedded (FFPE) samples (100% in OSCC and 78.5% in OPSCC), but reduced the sensitivity (33% in OSCC and 60% in OPSCC). The significant reduction of sensitivity by the double method was compensated by a very high sensitivity of p16-IHC detection in the triple approach.
Conclusions: Although HR-HPVs detection is of utmost importance in clinical settings for the Head and Neck Cancer patients, there is no consensus on which to consider the 'golden standard' among the numerous detection methods available either as single test or combinations. Until recently, quantitative E6 RNA PCR has been considered the 'golden standard' since it was demonstrated to have very high accuracy level and very high statistical significance associated with prognostic parameters. In contrast, quantitative E6 DNA PCR has proven to have very high level of accuracy but lesser prognostic association with clinical outcome than the HPV E6 oncoprotein RNA PCR. However, although it is theoretically possible to perform quantitative PCR detection methods also on FFPE samples, they reach the maximum of accuracy on fresh frozen tissue. Furthermore, worldwide diagnostic laboratories have not all the same ability to analyze simultaneously both FFPE and fresh tissues with these quantitative molecular detection methods. Therefore, in the current clinical practice a p16-IHC test is considered as sufficient for HPV diagnostic in accordance with the recently published Head and Neck Cancer international guidelines. Although p16-IHC may serve as a good prognostic indicator, our study clearly demonstrated that it is not satisfactory when used exclusively as the only HPV detecting method. Adding ISH, although known as less sensitive than PCR-based detection methods, has the advantage to preserve the morphological context of HPV-DNA signals in FFPE samples and, thus increase the overall specificity of p16/Consensus PCR combination tests.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

