Cholesterol level influences opioid signaling in cell models and analgesia in mice and humans
- PMID: 22377533
- PMCID: PMC3351822
- DOI: 10.1194/jlr.M024455
Cholesterol level influences opioid signaling in cell models and analgesia in mice and humans
Abstract
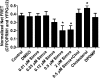
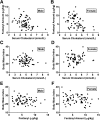
Cholesterol regulates the signaling of µ-opioid receptor in cell models, but it has not been demonstrated in mice or humans. Whether cholesterol regulates the signaling by mechanisms other than supporting the entirety of lipid raft microdomains is still unknown. By modulating cholesterol-enriched lipid raft microdomains and/or total cellular cholesterol contents in human embryonic kidney cells stably expressing µ-opioid receptor, we concluded that cholesterol stabilized opioid signaling both by supporting the lipid raft's entirety and by facilitating G protein coupling. Similar phenomena were observed in the primary rat hippocampal neurons. In addition, reducing the brain cholesterol level with simvastatin impaired the analgesic effect of opioids in mice, whereas the opioid analgesic effect was enhanced in mice fed a high-cholesterol diet. Furthermore, when the records of patients were analyzed, an inverse correlation between cholesterol levels and fentanyl doses used for anesthesia was identified, which suggested the mechanisms above could also be applicable to humans. Our results identified the interaction between opioids and cholesterol, which should be considered in clinics as a probable route for drug-drug interaction. Our studies also suggested that a low cholesterol level could lead to clinical issues, such as the observed impairment in opioid functions.
Figures





References
-
- Chini B., Parenti M. 2004. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J. Mol. Endocrinol. 32: 325–338 - PubMed
-
- Helms J. B., Zurzolo C. 2004. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 5: 247–254 - PubMed
-
- Ostrom R. S., Gregorian C., Drenan R. M., Xiang Y., Regan J. W., Insel P. A. 2001. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J. Biol. Chem. 276: 42063–42069 - PubMed
-
- Navratil A. M., Bliss S. P., Berghorn K. A., Haughian J. M., Farmerie T. A., Graham J. K., Clay C. M., Roberson M. S. 2003. Constitutive localization of the gonadotropin-releasing hormone (GnRH) receptor to low density membrane microdomains is necessary for GnRH signaling to ERK. J. Biol. Chem. 278: 31593–31602 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

